Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR2357) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
BRN-0183227
|
|||||
| Synonyms |
BRN 0183227; CHEBI:5070; CHEMBL274318; EINECS 207-654-8; MFCD00006841; MLS002667384; NSC 50393; NSC-50393; NSC50393; FLAVANONE; ZONYXWQDUYMKFB-UHFFFAOYSA-N; (-)-Flavanone; 2,3-Dihydro-2-phenyl-4H-1-benzopyran-4-one; 2,3-Dihydro-2-phenyl-4H-benzopyran-4-one; 2,3-Dihydroflavone; 2-Phenyl-2,3-dihydro-4H-chromen-4-one; 2-Phenyl-4-chromanone; 2-Phenyl-chroman-4-one; 2-Phenylchroman-4-one; 2-phenyl-2,3-dihydrochromen-4-one; 2-phenyl-3,4-dihydro-2H-1-benzopyran-4-one; 4-Flavanone; 487-26-3; 4H-1-Benzopyran-4-one, 2,3-dihydro-2-phenyl-; Flavanone
|
|||||
| Indication | Acute coronary syndrome [ICD11: BA4Z] | Phase 1 | [1] | |||
| Structure |
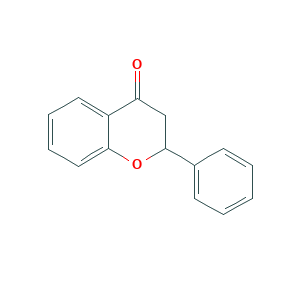 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 224.25 | Topological Polar Surface Area | 26.3 | ||
| Heavy Atom Count | 17 | Rotatable Bond Count | 1 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 2 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

