Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR3437) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Savolitinib
|
|||||
| Synonyms |
1313725-88-0; AZD-6094; AZD6094; UNII-2A2DA6857R; CHEMBL3334567; 2A2DA6857R; Savolitinib [INN]; Volitinib(Savolitinib); Savolitinib [USAN:INN]; GTPL9918; SCHEMBL12489208; EX-A845; BDBM50023342; ZINC149738712; AKOS030526403; DB12048; compound 28 [PMID: 25148209]; HY-15959; AS-35250; 1H-1,2,3-Triazolo(4,5-b)pyrazine, 1-((1S)-1-imidazo(1,2-a)pyridin-6-ylethyl)-6-(1-methyl-1H-pyrazol-4-yl)-; KB-333895; FT-0700162; J-690125; 4-{1-[(1S)-1-{imidazo[1,2-a]pyri
|
|||||
| Indication | Renal cell carcinoma [ICD11: 2C90] | Phase 3 | [1] | |||
| Solid tumour/cancer [ICD11: ICD11: 2A00-2F9Z] | Phase 2 | [2] | ||||
| Structure |
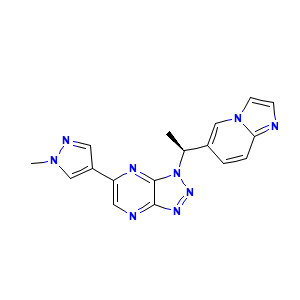 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 345.4 | Topological Polar Surface Area | 91.6 | ||
| Heavy Atom Count | 26 | Rotatable Bond Count | 3 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 6 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

