| Synonyms |
Sarasar; Lonafarnib [USAN]; Sch 66336; Sch66336; SCH-066336; Sch-66336; Lonafarnib (USAN/INN); (+)-4-(2-(4-(11R)-3,10-Dibromo-8-chloro-6,11-dihydro-5H-benzo(5,6)cyclohepta(1,2-b)pyridin-11-yl)-piperidin-1-yl))-2-oxoethyl)-piperidine-1-carboxamide; (+)-4-(2-(4-(8-chloro-3,10-dibromo-6,11-dihydro-5H-benzo(5,6)cyclohepta(1,2-b)pyridin-11-yl)-1-piperidinyl)-2-oxoethyl)-1-piperidinecarboxamide; (+)-4[2-[4-(8-Chloro-3,10-dibromo-6,11-dihydro-5H-benzo[5,6] cyclohepta[1,2-b]-pyridin-11(R)-yl-1-piperidinyl]-2-oxo-ethyl]-1-piperidinecarboxamide; 4-(2-(4-(8-Chloro-3,10-dibromo-6,11-dihydro-5H-benzo(5,6)cyclohepta(1,2-b)pyridin-11-yl)-1-piperidinyl)-2-oxoethyl)-1-piperidinecarboxamide; 4-(2-(4-(8-Chloro-3,10-dibromo-6,11-dihydro-5H-benzo-(5,6)-cyclohepta(1,2-b)-pyridin-11(R)-yl)-1-piperidinyl)-2-oxo-ethyl)-1-piperidinecarboxamide; 4-{2-[4-(3,10-DIBROMO-8-CHLORO-6,11-DIHYDRO-5H-BENZO[5,6]CYCLOHEPTA[1,2-B]PYRIDIN-11-YL)PIPERIDIN-1-YL]-2-OXOETHYL}PIPERIDINE-1-CARBOXAMIDE
|
| Cross-matching ID |
- PubChem CID
- 148195
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0W4HZ
- Formula
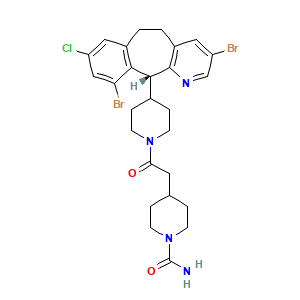
- C27H31Br2ClN4O2
- Canonical SMILES
- C1CN(CCC1CC(=O)N2CCC(CC2)[C@@H]3C4=C(CCC5=C3N=CC(=C5)Br)C=C(C=C4Br)Cl)C(=O)N
- InChI
- InChI=1S/C27H31Br2ClN4O2/c28-20-12-19-2-1-18-13-21(30)14-22(29)24(18)25(26(19)32-15-20)17-5-9-33(10-6-17)23(35)11-16-3-7-34(8-4-16)27(31)36/h12-17,25H,1-11H2,(H2,31,36)/t25-/m1/s1
- InChIKey
- DHMTURDWPRKSOA-RUZDIDTESA-N
|