| Synonyms |
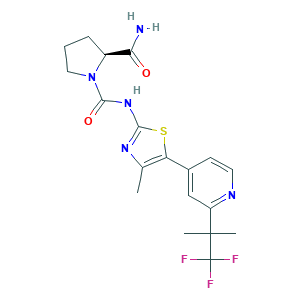
Alpelisib (BYL719); NVP-BYL719; alpelisib; (2S)-N1-[4-Methyl-5-[2-(2,2,2-trifluoro-1,1-dimethylethyl)-4-pyridinyl]-2-thiazolyl]-1,2-pyrrolidinedicarboxamide; (S)-N1-(4-Methyl-5-(2-(1,1,1-trifluoro-2-methylpropan-2-yl)pyridin-4-yl)thiazol-2-yl)pyrrolidine-1,2-dicarboxamide; 08W5N2C97Q; 1,2-Pyrrolidinedicarboxamide, N1-(4-methyl-5-(2-(2,2,2-trifluoro-1,1-dimethylethyl)-4-pyridinyl)-2-thiazolyl)-, (2S)-; 1217486-61-7; BYL 719; BYL-719; BYL719; C19H22F3N5O2S; CHEMBL2396661; UNII-08W5N2C97Q
|
| Cross-matching ID |
- PubChem CID
- 56649450
- PubChem SID
-
134426754
; 140722288
; 152159632
; 152255399
; 152258655
; 160647491
; 162012035
; 163643246
; 164041693
; 172918746
; 174486688
; 174861534
; 175448776
; 176251621
; 185965275
; 198967672
; 215785718
; 223382725
; 223626250
; 225848216
; 228076739
; 244202215
; 249565638
; 252214078
; 252451646
; 252470926
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0W7HE
- Formula
- C19H22F3N5O2S
- Canonical SMILES
- CC1=C(SC(=N1)NC(=O)N2CCCC2C(=O)N)C3=CC(=NC=C3)C(C)(C)C(F)(F)F
- InChI
- 1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1
- InChIKey
- STUWGJZDJHPWGZ-LBPRGKRZSA-N
|