| General Information of Drug (ID:
DR0098) |
| Drug Name |
Amlodipine besylate
|
| Synonyms |
Amcard; Amdepin; Amdipin; Amlodin; Amlodipine; Amlodipine (besylate); Amlodipine benzenesulfonate; Amlodipine besylate (Norvasc); Amlodipine besylate [USAN]; Amlogard; Amlopin; Amlosyn; Antacal; Astudar; CHEBI:2669; Cardiorex; Cordarene; HSDB 7079; Monopina; Myodura; NCGC00095835-01; Norlopin; Norvas; Norvask; Pelmec; Tensivask; Terloc; UK 48340-26; Amlocard; Amlodipine Free Base; Amlodipine [INN:BAN]; Amlodipino; Amlodipino [Spanish]; Amlodipinum; Amlodipinum [Latin]; Amlodis; Amlor; CHEBI:2668; Caduet; Coroval; HTIQEAQVCYTUBX-UHFFFAOYSA-N; Lipinox; Norvasc; UK-4834011; amlodipine; (s)-(-)-amlodipine; 3-ethyl 5-methyl 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate; 88150-42-9; AMLODIPINE BASE; 111470-99-6; 3-ethyl 5-methyl 2-((2-aminoethoxy)methyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate benzenesulfonate; AMLODIPINE BESYLATE
|
| Indication |
Essential hypertension
[ICD11: BA00]
|
Approved
|
[1]
|
| Structure |
|
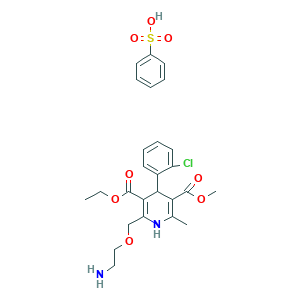 |
|
3D MOL is unavailable
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
567.1 |
Topological Polar Surface Area |
163 |
| Heavy Atom Count |
38 |
Rotatable Bond Count |
11 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
10 |
| Cross-matching ID |
- PubChem CID
- 60496
- PubChem SID
-
7847681
; 8186890
; 11363467
; 11366029
; 11368591
; 11376753
; 11494387
; 11528718
; 14837689
; 24890999
; 26612823
; 26681028
; 26749852
; 43117888
; 49830358
; 56320988
; 56423826
; 57314022
; 74532315
; 85174483
; 87246412
; 88802066
; 92308178
; 92711324
; 103770385
; 104320906
; 105807462
; 117633239
; 118048672
; 118843684
; 119526885
; 124637367
; 124757512
; 124800074
; 124801083
; 125164316
; 125307717
; 125337849
; 126630976
; 126657421
; 126665382
; 129990383
; 131294547
; 135016978
; 135692287
; 135698252
; 137241534
; 144075783
; 144115369
; 144205078
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0H7WA
- Formula
- C26H31ClN2O8S
- Canonical SMILES
- CCOC(=O)C1=C(NC(=C(C1C2=CC=CC=C2Cl)C(=O)OC)C)COCCN.C1=CC=C(C=C1)S(=O)(=O)O
- InChI
- 1S/C20H25ClN2O5.C6H6O3S/c1-4-28-20(25)18-15(11-27-10-9-22)23-12(2)16(19(24)26-3)17(18)13-7-5-6-8-14(13)21;7-10(8,9)6-4-2-1-3-5-6/h5-8,17,23H,4,9-11,22H2,1-3H3;1-5H,(H,7,8,9)
- InChIKey
- ZPBWCRDSRKPIDG-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


