| General Information of Drug (ID:
DR0102) |
| Drug Name |
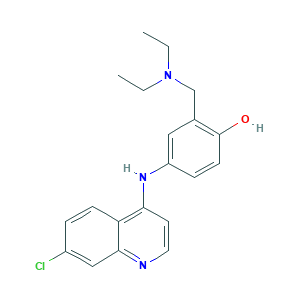
Amodiaquine
|
| Synonyms |
Amodiachin; Amodiachinum; Amodiaquin; Amodiaquina; Amodiaquina [INN-Spanish]; Amodiaquine USP24; Amodiaquine hydrochloride; Amodiaquine, ring-closed; Amodiaquinum; Amodiaquinum [INN-Latin]; CAM-AQI; Cam-AQ1; Camochin; Camoquin; Camoquin Hcl; Camoquinal; Camoquine; Flavoquine; Miaquin; S. N. 10751; SN 10,751; amodiaquine; 4-[(7-CHLOROQUINOLIN-4-YL)AMINO]-2-[(DIETHYLAMINO)METHYL]PHENOL; 86-42-0; C20H22ClN3O; CHEBI:2674; NSC 13453; Phenol, 4-[(7-chloro-4-quinolinyl)amino]-2-[(diethylamino)methyl]-; UNII-220236ED28
|
| Indication |
Malaria
[ICD11: 1F40]
|
Phase 4
|
[1]
|
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
355.9 |
Topological Polar Surface Area |
48.4 |
| Heavy Atom Count |
25 |
Rotatable Bond Count |
6 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 2165
- PubChem SID
-
9828
; 77901
; 598064
; 4621370
; 7886759
; 7978694
; 8151472
; 10537079
; 10589213
; 11466337
; 11467457
; 11486233
; 15374079
; 17397079
; 29221344
; 46393825
; 46506940
; 47291303
; 47440457
; 47589170
; 47810953
; 47959956
; 48415557
; 49698430
; 49956414
; 50085969
; 53789783
; 53801183
; 57321182
; 76179602
; 81062056
; 81093107
; 84974934
; 92712423
; 103190631
; 103973209
; 104299796
; 105086070
; 124596739
; 124659480
; 124801466
; 124951249
; 125358735
; 126630109
; 126678259
; 128866127
; 131333557
; 134337988
; 134970939
; 135702891
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D04NQI
- Formula
- C20H22ClN3O
- Canonical SMILES
- CCN(CC)CC1=C(C=CC(=C1)NC2=C3C=CC(=CC3=NC=C2)Cl)O
- InChI
- 1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23)
- InChIKey
- OVCDSSHSILBFBN-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


