| Synonyms |
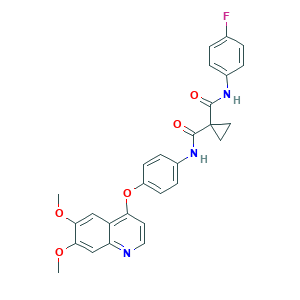
Cabozantinib; Cabozantinib (XL-184); Cabozantinib (XL184, BMS-907351); Cometriq; Cometriq (TN); XL 184; XL-184; 1,1-Cyclopropanedicarboxamide,N-[4-[(6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-N'-(4-fluorophenyl)-; 1C39JW444G; 849217-68-1; BMS 907351; BMS-907351; BMS907351; C28H24FN3O5; CHEBI:72317; N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide; UNII-1C39JW444G; XL184; n-(4-((6,7-dimethoxy-4-quinolinyl)oxy)phenyl)-n'-(4-fluorophenyl)-1,1-cyclopropanedicarboxamide
|
| Cross-matching ID |
- PubChem CID
- 25102847
- PubChem SID
-
103932275
; 117695999
; 131480693
; 135267839
; 135626782
; 135727427
; 136367316
; 136368016
; 136920380
; 137256574
; 137276008
; 137760418
; 143499870
; 144115772
; 152234431
; 152258418
; 160647254
; 160676622
; 160962864
; 162011361
; 162037456
; 162202657
; 164023425
; 164041945
; 164837657
; 172914677
; 174530916
; 175266754
; 178102511
; 185997013
; 198993077
; 202543085
; 223387911
; 224083495
; 226693608
; 244920971
; 249737079
; 249896088
; 252088620
; 252110119
; 252158839
; 252215083
; 252439619
; 252451817
; 252543409
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0IQ6P
- Formula
- C28H24FN3O5
- Canonical SMILES
- COC1=CC2=C(C=CN=C2C=C1OC)OC3=CC=C(C=C3)NC(=O)C4(CC4)C(=O)NC5=CC=C(C=C5)F
- InChI
- 1S/C28H24FN3O5/c1-35-24-15-21-22(16-25(24)36-2)30-14-11-23(21)37-20-9-7-19(8-10-20)32-27(34)28(12-13-28)26(33)31-18-5-3-17(29)4-6-18/h3-11,14-16H,12-13H2,1-2H3,(H,31,33)(H,32,34)
- InChIKey
- ONIQOQHATWINJY-UHFFFAOYSA-N
|