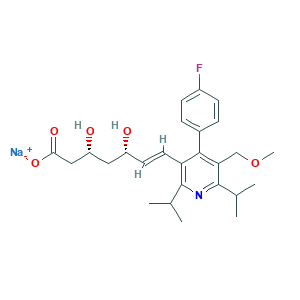
| Synonyms |
Cerivastatin sodium [USAN]; Cerivastatin, sodium salt; Certa; DSSTox_CID_26488; DSSTox_GSID_46488; DSSTox_RID_81659; Rivastatin; UNII-6Q18G1060S; Cerivastatin; Cerivastatin [INN:BAN]; HSDB 7357; UNII-AM91H2KS67; cerivastatin; cerivastatin acid; (3R,5S,6E)-7-(4-(4-Fluorophenyl)-5-(methoxymethyl)-2,6-bis(1-methylethyl)-3-pyridinyl)-3,5-dihydroxy-6-heptenoic acid; (3R,5S,6E)-7-(4-(p-Fluorophenyl)-2,6-diisopropyl-5-(methoxymethyl)-3-pyridyl)-3,5-dihydroxy-6-heptenoic acid; 145599-86-6; 6-Heptenoic acid, 7-(4-(4-fluorophenyl)-5-(methoxymethyl)-2,6-bis(1-methylethyl)-3-pyridinyl)-3,5-dihydroxy-, (3R,5S,6E)-; AM91H2KS67; CHEBI:3558; (+)-Sodium (3R,5S,6E)-7-(4-(p-fluorophenyl)-2,6-diisopropyl-5-(methoxymethyl)-3-pyridyl)-3,5-dihydroxy-6-heptenoate; 143201-11-0; 6Q18G1060S; 7-(4-(4-Fluorophenyl)-2,6-diisopropyl-5-(methoxymethyl)pyrid-3-yl)-3,5-dihydroxy-6-heptenoate sodium salt; Bay w 6228; Bay-w-6228; CERIVASTATIN Na; CERIVASTATIN SODIUM; CHEBI:3559; CPD000469148
|
| Cross-matching ID |
- PubChem CID
- 23663992
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D03KIA
- Formula
- C26H33FNNaO5
- Canonical SMILES
- CC(C)C1=C(C(=C(C(=N1)C(C)C)COC)C2=CC=C(C=C2)F)C=CC(CC(CC(=O)[O-])O)O.[Na+]
- InChI
- 1S/C26H34FNO5.Na/c1-15(2)25-21(11-10-19(29)12-20(30)13-23(31)32)24(17-6-8-18(27)9-7-17)22(14-33-5)26(28-25)16(3)4;/h6-11,15-16,19-20,29-30H,12-14H2,1-5H3,(H,31,32);/q;+1/p-1/b11-10+;/t19-,20-;/m1./s1
- InChIKey
- GPUADMRJQVPIAS-QCVDVZFFSA-M
|