| General Information of Drug (ID:
DR0627) |
| Drug Name |
Esomeprazole
|
| Prodrug Info |
Esomeprazole is the prodrug of 5-hydroxyomeprazole
|
| Synonyms |
Esomeprazole (INN); Esomeprazole Sodium; Esomeprazole [INN:BAN]; Inexium paranova; Inexium paranova (TN); Nexiam; Nexium; esomeprazol; esomeprazolum; Alenia; Esofag; (-)-Omeprazole; (S)-(-)-Omeprazole; (S)-5-Methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfinyl]-1H-benzimidazole; (S)-6-Methoxy-2-(((4-methoxy-3,5-dimethylpyridin-2-yl)methyl)sulfinyl)-1H-benzo[d]imidazole; (S)-Esomeprazole; (S)-Omeprazole; 119141-88-7; CHEBI:50275; Escz; N3PA6559FT; UNII-N3PA6559FT
|
| Indication |
Duodenal ulcer
[ICD11: DA63]
|
Approved
|
[1]
|
| Structure |
|
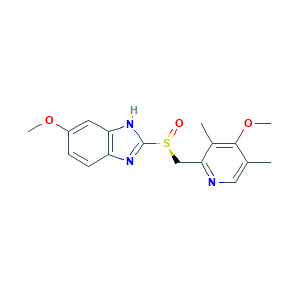 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
345.4 |
Topological Polar Surface Area |
96.3 |
| Heavy Atom Count |
24 |
Rotatable Bond Count |
5 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
6 |
| Cross-matching ID |
- PubChem CID
- 9568614
- PubChem SID
-
591076
; 14875947
; 14900571
; 44707139
; 56394969
; 80295315
; 93166178
; 96024613
; 124759673
; 124968856
; 126525308
; 126670961
; 129288902
; 129288903
; 137001228
; 137752931
; 139533883
; 152040240
; 160825217
; 163092495
; 163384365
; 163840367
; 164044845
; 175267781
; 175269116
; 175437905
; 178102135
; 189657728
; 198993898
; 204429634
; 211535433
; 223971225
; 226409261
; 246378786
; 249855831
; 250200763
; 251886328
; 251964001
; 252074449
; 252430137
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0C6DT
- Formula
- C17H19N3O3S
- Canonical SMILES
- CC1=CN=C(C(=C1OC)C)CS(=O)C2=NC3=C(N2)C=C(C=C3)OC
- InChI
- 1S/C17H19N3O3S/c1-10-8-18-15(11(2)16(10)23-4)9-24(21)17-19-13-6-5-12(22-3)7-14(13)20-17/h5-8H,9H2,1-4H3,(H,19,20)/t24-/m0/s1
- InChIKey
- SUBDBMMJDZJVOS-DEOSSOPVSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


