| Synonyms |
Fluindostatin sodium; Fluvastatin (sodium); Fluvastatin sodium (Lescol); Fluvastatin sodium salt; Fluyastatin Sodium Salt; Fractal; Lescol XL; Lipaxan; Lochol; Prestwick_1032; Sri 62320; Sri-62320; Vastin; XU 62-320; XU-62-320; Xilep XL; 93957-55-2; 94061-80-0; AB01274723-01; C24H25FNNaO4; CAS-93957-55-2; CHEBI:77602; Canef; DSSTox_CID_24758; DSSTox_GSID_44758; DSSTox_RID_80451; Locol; SMR000550480; Sodium (3R,5S,E)-7-(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl)-3,5-dihydroxyhept-6-enoate; FLUVASTATIN SODIUM; Fluvastatin [INN:BAN]; XU 62320; XU-62320; fluvastatin; (+)-(3R,5S)-fluvastatin; (3R,5S)-fluvastatin; (3R,5S,6E)-7-[3-(4-fluorophenyl)-1-(1-methylethyl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoic acid; (3R,5S,6E)-7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoic acid; (3R,5S,6E)-7-[3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoic acid; 93957-54-1; C24H26FNO4; CHEBI:38565; EN300-51915; CHEMBL1078
|
| Cross-matching ID |
- PubChem CID
- 23663976
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D08GHB
- Formula
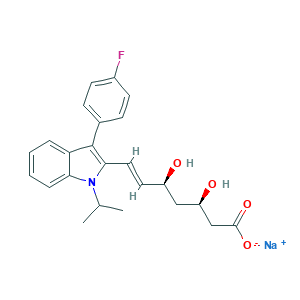
- C24H25FNNaO4
- Canonical SMILES
- CC(C)N1C2=CC=CC=C2C(=C1C=CC(CC(CC(=O)[O-])O)O)C3=CC=C(C=C3)F.[Na+]
- InChI
- 1S/C24H26FNO4.Na/c1-15(2)26-21-6-4-3-5-20(21)24(16-7-9-17(25)10-8-16)22(26)12-11-18(27)13-19(28)14-23(29)30;/h3-12,15,18-19,27-28H,13-14H2,1-2H3,(H,29,30);/q;+1/p-1/b12-11+;/t18-,19-;/m1./s1
- InChIKey
- ZGGHKIMDNBDHJB-NRFPMOEYSA-M
|