| General Information of Drug (ID:
DR0833) |
| Drug Name |
Hydromorphone
|
| Synonyms |
Hydromorfona; Hydromorfona [Spanish]; Hydromorphon; Hydromorphone [INN:BAN]; Hydromorphonum; Hydromorphonum [INN-Latin]; Idromorfone; Idromorfone [DCIT]; Laudacon; Laudicon; Morphinone, dihydro-; Novolaudon; Palladone; hydromorphone; Dihydromorfinon; Dihydromorfinon [Czech]; Dihydromorphinone; Dilaudid; Dilaudid Oros; Dilaudid-hp; Dimorphone; Hidromorfona; Hidromorfona [INN-Spanish]; 4,5-Epoxy-3-hydroxy-17-methylmorphinan-6-one; 466-99-9; 6-Deoxy-7,8-dihydro-6-oxomorphine; 7,8-Dihydromorphinone; DiMo
|
| Indication |
Anaesthesia
[ICD11: 8E22]
|
Approved
|
[1]
|
| Structure |
|
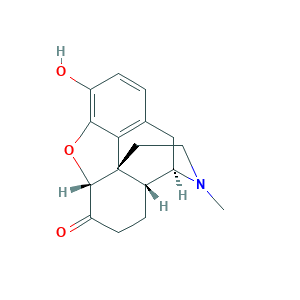 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
285.34 |
Topological Polar Surface Area |
49.8 |
| Heavy Atom Count |
21 |
Rotatable Bond Count |
0 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 5284570
- PubChem SID
-
9254
; 81990
; 7979546
; 11039353
; 14751077
; 14873177
; 39317885
; 46508700
; 48416092
; 49993162
; 50070721
; 56394822
; 57359121
; 77568926
; 96024740
; 103557031
; 104098728
; 129640116
; 134222944
; 134337767
; 134974682
; 137005111
; 139157539
; 160963675
; 175268533
; 178103661
; 179116727
; 184536600
; 198992261
; 223798795
; 226394766
; 250134622
; 252350812
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D04JHN
- Formula
- C17H19NO3
- Canonical SMILES
- CN1CCC23C4C1CC5=C2C(=C(C=C5)O)OC3C(=O)CC4
- InChI
- 1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2,4,10-11,16,19H,3,5-8H2,1H3/t10-,11+,16-,17-/m0/s1
- InChIKey
- WVLOADHCBXTIJK-YNHQPCIGSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


