| Synonyms |
Levomilnacipran; Levomilnacipran (USAN/INN); Levomilnacipran [USAN:INN]; Milnacipram; SB17447; SCHEMBL1414867; UGM0326TXX; ZINC506; (+)-Milnacipran; Fetzima; GJJFMKBJSRMPLA-DZGCQCFKSA-N; (1S,2R)-2-(aminomethyl)-N,N-diethyl-1-phenylcyclopropane-1-carboxamide; (1S,2R)-2-(aminomethyl)-N,N-diethyl-1-phenylcyclopropanecarboxamide; (1S,2R)-milnacipran; 96847-54-0; 96847-55-1; AC1OCEN8; BDBM50032379; CHEBI:136040; CHEMBL99946; D10072; DB08918; F 2695; F-2695; GTPL7435; UNII-UGM0326TXX; f2-695
|
| Cross-matching ID |
- PubChem CID
- 6917779
- PubChem SID
-
16010452
; 23950786
; 43529203
; 50872352
; 103316413
; 103940908
; 114786948
; 127962304
; 135626792
; 137129347
; 139020085
; 160658357
; 175271225
; 175427154
; 178104007
; 179149463
; 198967714
; 227628532
; 250148599
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D02XOK
- Formula
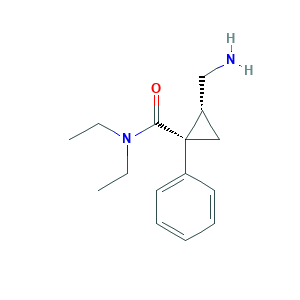
- C15H22N2O
- Canonical SMILES
- CCN(CC)C(=O)C1(CC1CN)C2=CC=CC=C2
- InChI
- 1S/C15H22N2O/c1-3-17(4-2)14(18)15(10-13(15)11-16)12-8-6-5-7-9-12/h5-9,13H,3-4,10-11,16H2,1-2H3/t13-,15+/m0/s1
- InChIKey
- GJJFMKBJSRMPLA-DZGCQCFKSA-N
|