| Synonyms |
Loratinib; Lorbrena; Lorlatinib; Lorlatinib,PF-06463922; OSP71S83EU; PF 06463922; PF-06463922; PF06463922; (10R)-7-amino-12-fluoro-10,15,16,17-tetrahydro-2,10,16-trimethyl-15-oxo-2H-4,8-methenopyrazolo[4,3-h][2,5,11]benzoxadiazacyclotetradecine-3-carbonitrile; (10r)-7-Amino-12-Fluoro-2,10,16-Trimethyl-15-Oxo-10,15,16,17-Tetrahydro-2h-8,4-(Metheno)pyrazolo[4,3-H][2,5,11]benzoxadiazacyclotetradecine-3-Carbonitrile; 1454846-35-5; CHEMBL3286830; MFCD28144520; UNII-OSP71S83EU
|
| Cross-matching ID |
- PubChem CID
- 71731823
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0AF6O
- Formula
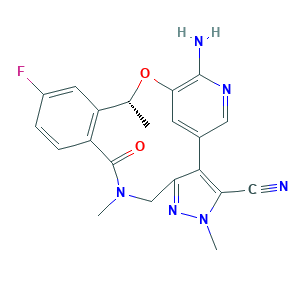
- C21H19FN6O2
- Canonical SMILES
- CC1C2=C(C=CC(=C2)F)C(=O)N(CC3=NN(C(=C3C4=CC(=C(N=C4)N)O1)C#N)C)C
- InChI
- 1S/C21H19FN6O2/c1-11-15-7-13(22)4-5-14(15)21(29)27(2)10-16-19(17(8-23)28(3)26-16)12-6-18(30-11)20(24)25-9-12/h4-7,9,11H,10H2,1-3H3,(H2,24,25)/t11-/m1/s1
- InChIKey
- IIXWYSCJSQVBQM-LLVKDONJSA-N
|