| General Information of Drug (ID:
DR1051) |
| Drug Name |
Methyldopa
|
| Synonyms |
Medomet; Medopa; Medopal; Medopren; Methoplain; Methyl dopa; Methyl-L-dopa; Methyldopa anhydrous; Methyldopum; Metildopa; Presinol; Aldomet; Aldometil; Aldomin; Alpha medopa; Alphamethyldopa; Baypresol; Becanta; Dopamet; Dopamethyperpax; Dopatec; Dopegit; Dopegyt; Grospisk; Hyperpax; Hypolag; L-(alpha-Md); L-Methyl Dopa; L-alpha-Methyl DOPA; Presolisin; Sedometil; Sembrina; alpha-Methyl dopa; alpha-Methyldopa; l-alpha-Methyldopa; methyldopa; (S)-(-)-alpha-Methyldopa; 3-(3,4-DIHYDROXYPHENYL)-2-METHYL-L-ALANINE; 555-30-6
|
| Indication |
Essential hypertension
[ICD11: BA00]
|
Approved
|
[1]
|
| Structure |
|
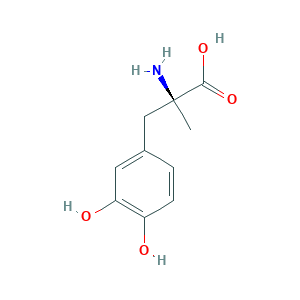 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
211.21 |
Topological Polar Surface Area |
104 |
| Heavy Atom Count |
15 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
4 |
Hydrogen Bond Acceptor Count |
5 |
| Cross-matching ID |
- PubChem CID
- 38853
- PubChem SID
-
9403
; 597355
; 7979942
; 8139902
; 8149423
; 8175761
; 10321684
; 11113581
; 11335317
; 11360556
; 11363831
; 11366393
; 11368955
; 11371621
; 11373686
; 11377117
; 11461528
; 11466354
; 11467474
; 11484792
; 11485988
; 11488884
; 11490317
; 11491869
; 11494751
; 14748911
; 17405414
; 26611818
; 26679555
; 34704829
; 46508535
; 47290999
; 47440113
; 47736324
; 47736325
; 47810607
; 48034965
; 48110315
; 48184859
; 48416254
; 48423673
; 49698436
; 49854436
; 50104474
; 50104475
; 50104476
; 50104477
; 50970645
; 53777959
; 53790662
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0BA6T
- Formula
- C10H13NO4
- Canonical SMILES
- CC(CC1=CC(=C(C=C1)O)O)(C(=O)O)N
- InChI
- 1S/C10H13NO4/c1-10(11,9(14)15)5-6-2-3-7(12)8(13)4-6/h2-4,12-13H,5,11H2,1H3,(H,14,15)/t10-/m0/s1
- InChIKey
- CJCSPKMFHVPWAR-JTQLQIEISA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


