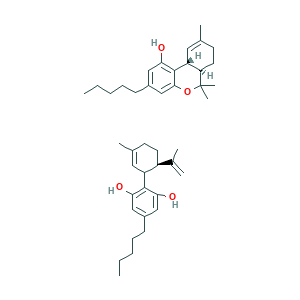
| Cross-matching ID |
- PubChem CID
- 44148067
- PubChem SID
-
135262436
; 202535631
- CAS Number
-
- TTD Drug ID
- D0C5CE
- Formula
- C42H60O4
- Canonical SMILES
- CCCCCC1=CC(=C(C(=C1)O)C2C=C(CCC2C(=C)C)C)O.CCCCCC1=CC(=C2C3C=C(CCC3C(OC2=C1)(C)C)C)O
- InChI
- 1S/2C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15;1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,16-17,22H,5-10H2,1-4H3;11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t16-,17-;17-,18?/m10/s1
- InChIKey
- SSNHGLKFJISNTR-FWUPRJFYSA-N
|