| General Information of Drug (ID:
DR1279) |
| Drug Name |
Phenprocoumon
|
| Synonyms |
Phenprocoumarol; Phenprocoumarole; Phenprocoumone; Phenprocoumone [INN-French]; Phenprocoumonum; Phenprocoumonum [INN-Latin]; Phenprocumone; Phenprocumonum; Ro 1-4849; 3-(1'-Phenyl-propyl)-4-oxycoumarin; 3-(1-Phenylpropyl)-4-hydroxycoumarin; Falithrom; Fencumar; Fenprocoumona [INN-Spanish]; Fenprocumone; Fenprocumone [DCIT]; Liquamar; Marcoumar; Marcumar; PHENPROCOUMON; 3-(alpha-Ethylbenzyl)-4-hydroxycoumarin; 4-Hydroxy-3-(1-phenylpropyl)-2H-1-benzopyran-2-one; 4-Hydroxy-3-(1-phenylpropyl)-2H-chromen-2-one; 435-97-2
|
| Indication |
Thrombosis
[ICD11: DB61]
|
Approved
|
[1]
|
| Structure |
|
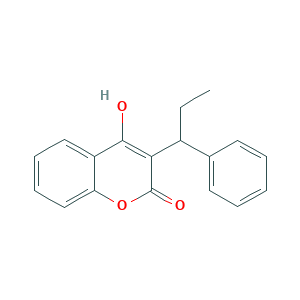 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
280.3 |
Topological Polar Surface Area |
46.5 |
| Heavy Atom Count |
21 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 54680692
- PubChem SID
-
612021
; 7845703
; 7980293
; 8030662
; 10522853
; 14848717
; 29228465
; 42684251
; 46506423
; 47207126
; 47815891
; 48416418
; 49956264
; 56394971
; 57309600
; 57325861
; 76965160
; 93302774
; 103179879
; 103950577
; 104253508
; 104321863
; 106673762
; 125823525
; 126658557
; 127770897
; 134338056
; 134974499
; 135692445
; 137126448
; 137239431
; 142971044
; 160964285
; 162011455
; 162224643
; 162652374
; 163418248
; 164042860
; 174529392
; 176262013
; 178103445
; 179326620
; 184545898
; 186014484
; 198993025
; 219400682
; 223670210
; 223705291
; 226427577
; 227840593
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0QV5T
- Formula
- C18H16O3
- Canonical SMILES
- CCC(C1=CC=CC=C1)C2=C(C3=CC=CC=C3OC2=O)O
- InChI
- 1S/C18H16O3/c1-2-13(12-8-4-3-5-9-12)16-17(19)14-10-6-7-11-15(14)21-18(16)20/h3-11,13,19H,2H2,1H3
- InChIKey
- DQDAYGNAKTZFIW-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


