| Synonyms |
Pirkam; Piroflex; Piroftal; Piroxicam (Feldene); Piroxicamum; Piroxicamum [INN-Latin]; Pyroxycam; Reudene; Riacen; Roxicam; Roxiden; Sasulen; Solocalm; Artroxicam; Bruxicam; CHF 1251; Caliment; Erazon; Feldene; Flogobene; Geldene; Improntal; Larapam; Zunden; piroxicam; piroxicam usp; 2H-1,2-Benzothiazine-3-carboxamide, 4-hydroxy-2-methyl-N-2-pyridinyl-, 1,1-dioxide; 36322-90-4; 4-Hydroxy-2-methyl-N-(pyridin-2-yl)-2H-benzo[e][1,2]thiazine-3-carboxamide 1,1-dioxide; BAXO; CCRIS 3719; CP 16171; CP-16171; NSC 666076; Roxam; UNII-13T4O6VMAM
|
| Cross-matching ID |
- PubChem CID
- 54676228
- PubChem SID
-
4761
; 511550
; 627224
; 856007
; 859011
; 3248708
; 5005569
; 7847195
; 7980333
; 8149487
; 10321546
; 10520247
; 10591135
; 11111641
; 11111642
; 11113322
; 11120243
; 11120731
; 11121219
; 11121700
; 11122180
; 11335544
; 11360783
; 11362784
; 11364009
; 11365346
; 11366571
; 11367908
; 11369133
; 11370819
; 11370820
; 11371780
; 11373509
; 11374454
; 11376070
; 11377295
; 11427267
; 11461755
; 11466239
; 11467359
; 11485029
; 11485878
; 11489103
; 11490596
; 11492783
; 11494929
; 12013846
; 14924343
; 17405458
; 24583179
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D00IBN
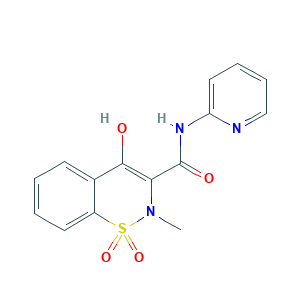
- Formula
- C15H13N3O4S
- Canonical SMILES
- CN1C(=C(C2=CC=CC=C2S1(=O)=O)O)C(=O)NC3=CC=CC=N3
- InChI
- 1S/C15H13N3O4S/c1-18-13(15(20)17-12-8-4-5-9-16-12)14(19)10-6-2-3-7-11(10)23(18,21)22/h2-9,19H,1H3,(H,16,17,20)
- InChIKey
- QYSPLQLAKJAUJT-UHFFFAOYSA-N
|