| Synonyms |
Pomalidomide; Pomalidomide (CC-4047); Pomalyst; Actimid; IMID-3; Imnovid; 19171-19-8; 1H-Isoindole-1,3(2H)-dione, 4-amino-2-(2,6-dioxo-3-piperidinyl)-; 3-Amino-N-(2,6-dioxo-3-piperidyl)phthalimide; 4-Amino-2-(2,6-dioxo-3-piperidyl)isoindoline-1,3-dione; 4-Aminothalidomide; 4-amino-2-(2,6-dioxopiperidin-3-yl)-1H-isoindole-1,3(2H)-dione; 4-amino-2-(2,6-dioxopiperidin-3-yl)isoindole-1,3-dione; 4-amino-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione; AK104087; CC 4047; CC-4047; CHEBI:72690
|
| Cross-matching ID |
- PubChem CID
- 134780
- PubChem SID
-
10243744
; 14848461
; 29312424
; 50086901
; 50447747
; 53787052
; 57345004
; 96025659
; 103100612
; 103221733
; 104253216
; 104382952
; 117621779
; 124757341
; 125164145
; 125697093
; 129427476
; 135089995
; 135727444
; 136348254
; 136367549
; 136946644
; 137009922
; 142523166
; 143499180
; 149539643
; 152235120
; 152258286
; 152343904
; 160647125
; 160837107
; 162011791
; 162012270
; 162037668
; 162189531
; 162856472
; 163398415
; 163778934
; 163907985
; 164194051
; 172889447
; 172889448
; 173130995
; 174007398
; 174528127
; 175424471
; 175427148
; 178103920
; 179116970
; 186007045
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0A3ZU
- Formula
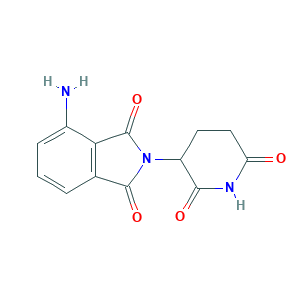
- C13H11N3O4
- Canonical SMILES
- C1CC(=O)NC(=O)C1N2C(=O)C3=C(C2=O)C(=CC=C3)N
- InChI
- 1S/C13H11N3O4/c14-7-3-1-2-6-10(7)13(20)16(12(6)19)8-4-5-9(17)15-11(8)18/h1-3,8H,4-5,14H2,(H,15,17,18)
- InChIKey
- UVSMNLNDYGZFPF-UHFFFAOYSA-N
|