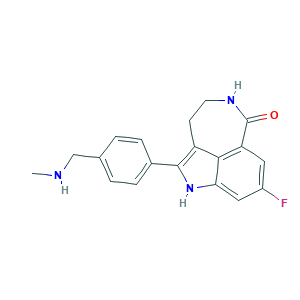
| Synonyms |
Rubraca; Rucaparib (free base); 283173-50-2; 6H-Pyrrolo[4,3,2-ef][2]benzazepin-6-one,8-fluoro-1,3,4,5-tetrahydro-2-[4-[(methylamino)methyl]phenyl]-; RUCAPARIB; 8-FLUORO-2-(4-((METHYLAMINO)METHYL)PHENYL)-4,5-DIHYDRO-1H-AZEPINO[5,4,3-CD]INDOL-6(3H)-ONE; 8-Fluoro-1,3,4,5-tetrahydro-2-[4-[(methylamino)methyl]phenyl]-6H-pyrrolo[4,3,2-ef][2]benzazepin-6-one; 8-fluoro-2-(4-methylaminomethyl-phenyl)-1,3,4,5-tetrahydro-azepino[5,4,3-cd]indol-6-one; 8237F3U7EH; AG-14447; AK317822; UNII-8237F3U7EH
|
| Cross-matching ID |
- PubChem CID
- 9931954
- PubChem SID
-
16463842
; 24207084
; 76934109
; 103769588
; 103905261
; 121278129
; 128080164
; 135626799
; 137263927
; 137276017
; 142270192
; 152037846
; 162108736
; 162829534
; 164208495
; 170474818
; 172918029
; 174006354
; 177748908
; 185992962
; 194944026
; 198980201
; 210274713
; 210280347
; 223366069
; 223471430
; 223804825
; 227116711
; 249625884
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D01SHZ
- Formula
- C19H18FN3O
- Canonical SMILES
- CNCC1=CC=C(C=C1)C2=C3CCNC(=O)C4=C3C(=CC(=C4)F)N2
- InChI
- 1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24)
- InChIKey
- HMABYWSNWIZPAG-UHFFFAOYSA-N
|