| General Information of Drug (ID:
DR1471) |
| Drug Name |
Selexipag
|
| Prodrug Info |
Selexipag is the prodrug of ACT-333679
|
| Synonyms |
Selexipag; Selexipag [USAN:INN]; Selexipag(NS-304); Uptravi; Uptravi (TN); 2-(4-((5,6-Diphenylpyrazin-2-yl)(propan-2-yl)amino)butoxy}-n-(methanesulfonyl)acetamide; 2-(4-((5,6-diphenylpyrazin-2-yl)(isopropyl)amino)butoxy)-N-(methylsulfonyl)acetamide; 2-{4-[(5,6-diphenylpyrazin-2-yl)(propan-2-yl)amino]butoxy}-N-(methanesulfonyl)acetamide; 2-{4-[N-(5,6-diphenylpyrazin-2-yl)-N-isopropylamino]butyloxy}-N-(methylsulfonyl)acetamide; 475086-01-2; 5EXC0E384L; ACT 293987; ACT-293987; NS 304; NS-304; UNII-5EXC0E384L
|
| Indication |
Pulmonary hypertension
[ICD11: BB01]
|
Approved
|
[1]
|
| Structure |
|
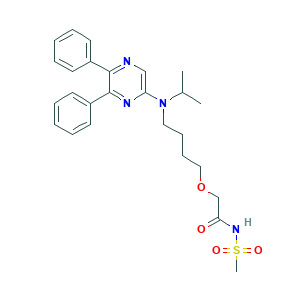 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
496.6 |
Topological Polar Surface Area |
110 |
| Heavy Atom Count |
35 |
Rotatable Bond Count |
12 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
7 |
| Cross-matching ID |
- PubChem CID
- 9913767
- PubChem SID
-
14884195
; 24193522
; 45985422
; 71826069
; 75827381
; 103543954
; 125335605
; 127344242
; 127344243
; 135268706
; 135626715
; 163125035
; 163893551
; 198953024
; 204362837
; 208265357
; 223365888
; 223872265
; 226969886
; 241376405
; 244974834
; 252157218
; 252228662
; 252480281
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0N2SR
- Formula
- C26H32N4O4S
- Canonical SMILES
- CCOC1=C(C=C2C(=C1)N=CC(=C2NC3=CC(=C(C=C3)OCC4=CC=CC=N4)Cl)C#N)NC(=O)C=CCN(C)C
- InChI
- 1S/C26H32N4O4S/c1-20(2)30(16-10-11-17-34-19-24(31)29-35(3,32)33)23-18-27-25(21-12-6-4-7-13-21)26(28-23)22-14-8-5-9-15-22/h4-9,12-15,18,20H,10-11,16-17,19H2,1-3H3,(H,29,31)
- InChIKey
- QXWZQTURMXZVHJ-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


