| General Information of Drug (ID:
DR1482) |
| Drug Name |
Silodosin
|
| Synonyms |
Silodosin; Silodosin-d6; Silodyx; Urorec; CUZ39LUY82; KAD 3213; KAD-3213; KMD 3213; KMD-3213; Q-102517; Rapaflo; Rapflo; (R)-1-(3-hydroxypropyl)-5-(2-((2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)amino)propyl)indoline-7-carboxamide; 1-(3-hydroxypropyl)-5-[(2R)-2-[2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethylamino]propyl]-2,3-dihydroindole-7-carboxamide; 160970-54-7; 160970-64-9; CHEMBL24778; UNII-CUZ39LUY82; Urief
|
| Indication |
Prostatic hyperplasia
[ICD11: GA90]
|
Approved
|
[1]
|
| Structure |
|
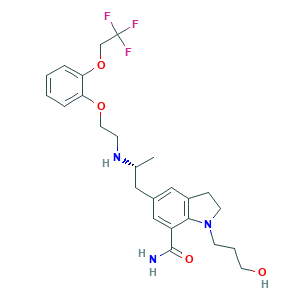 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
495.5 |
Topological Polar Surface Area |
97 |
| Heavy Atom Count |
35 |
Rotatable Bond Count |
13 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
9 |
| Cross-matching ID |
- PubChem CID
- 5312125
- PubChem SID
-
7849027
; 7979683
; 11056891
; 12014894
; 14810706
; 14835413
; 39341745
; 57359519
; 80176928
; 92098546
; 99299508
; 103194366
; 104046714
; 109693099
; 114157936
; 126623000
; 126665711
; 128939931
; 131300819
; 134339064
; 134340151
; 134342132
; 135252710
; 135368407
; 135650458
; 135916576
; 137248544
; 142093997
; 152258092
; 152344280
; 160646931
; 162011672
; 162172235
; 162223757
; 163884568
; 164765008
; 164777753
; 172085148
; 174527805
; 175266679
; 175427058
; 175612190
; 179150028
; 184816547
; 187071911
; 198991703
; 202821102
; 211536181
; 223392962
; 223484371
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0U0VU
- Formula
- C25H32F3N3O4
- Canonical SMILES
- CC(CC1=CC2=C(C(=C1)C(=O)N)N(CC2)CCCO)NCCOC3=CC=CC=C3OCC(F)(F)F
- InChI
- 1S/C25H32F3N3O4/c1-17(30-8-12-34-21-5-2-3-6-22(21)35-16-25(26,27)28)13-18-14-19-7-10-31(9-4-11-32)23(19)20(15-18)24(29)33/h2-3,5-6,14-15,17,30,32H,4,7-13,16H2,1H3,(H2,29,33)/t17-/m1/s1
- InChIKey
- PNCPYILNMDWPEY-QGZVFWFLSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


