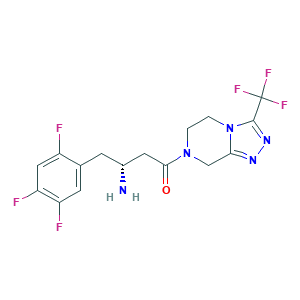
| Synonyms |
Sitagliptan; Sitagliptin; Sitagliptin phosphate; Tesavel; Xelevia; Januvia; MK-0431; QFP0P1DV7Z; (2R)-4-OXO-4-[3-(TRIFLUOROMETHYL)-5,6-DIHYDRO[1,2,4]TRIAZOLO[4,3-A]PYRAZIN-7(8H)-YL]-1-(2,4,5-TRIFLUOROPHENYL)BUTAN-2-AMINE; (3R)-3-AMINO-1-[3-(TRIFLUOROMETHYL)-5H,6H,7H,8H-[1,2,4]TRIAZOLO[4,3-A]PYRAZIN-7-YL]-4-(2,4,5-TRIFLUOROPHENYL)BUTAN-1-ONE; (R)-3-AMINO-1-(3-(TRIFLUOROMETHYL)-5,6-DIHYDRO-[1,2,4]TRIAZOLO[4,3-A]PYRAZIN-7(8H)-YL)-4-(2,4,5-TRIFLUOROPHENYL)BUTAN-1-ONE; 486460-32-6; CHEBI:40237; UNII-QFP0P1DV7Z
|
| Cross-matching ID |
- PubChem CID
- 4369359
- PubChem SID
-
7885474
; 9656038
; 14904024
; 16466401
; 35668584
; 46393524
; 46505822
; 46511716
; 46513917
; 46530623
; 48034814
; 50070925
; 56365842
; 78231257
; 85789641
; 92307950
; 96025201
; 99443685
; 103502876
; 112947540
; 117695427
; 126669712
; 126731480
; 134337929
; 135206675
; 136339733
; 137005783
; 143037551
; 143497677
; 152028143
; 152164204
; 152164205
; 152238548
; 152344340
; 160646743
; 160964592
; 162201432
; 164784279
; 164824529
; 164846813
; 165235282
; 165245552
; 165702336
; 170499559
; 174006349
; 174529510
; 175268642
; 178102906
; 179231014
; 186005103
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0U2JP
- Formula
- C16H15F6N5O
- Canonical SMILES
- C1CN2C(=NN=C2C(F)(F)F)CN1C(=O)CC(CC3=CC(=C(C=C3F)F)F)N
- InChI
- 1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1
- InChIKey
- MFFMDFFZMYYVKS-SECBINFHSA-N
|