| General Information of Drug (ID:
DR1536) |
| Drug Name |
Tazarotene
|
| Prodrug Info |
Tazarotene is the prodrug of Tazarotenic acid
|
| Synonyms |
Tazarotene (Avage); Tazarotene [USAN:INN]; Tazorac; tazarotene; 118292-40-3; 3-Pyridinecarboxylic acid, 6-((3,4-dihydro-4,4-dimethyl-2H-1-benzothiopyran-6-yl)ethynyl)-, ethyl ester; Fabior; OGQICQVSFDPSEI-UHFFFAOYSA-N; 81BDR9Y8PS; AGN 190168; AGN-190168; Avage; C21H21NO2S; CHEBI:32184; CHEMBL1657; Ethyl 6-((4,4-dimethylthiochroman-6-yl)ethynyl)nicotinate; NCGC00167525-01; UNII-81BDR9Y8PS; Zora; Zorac; ethyl 6-[2-(4,4-dimethylthiochroman-6-yl)ethynyl]pyridine-3-carboxylate
|
| Indication |
Psoriasis vulgaris
[ICD11: EA90]
|
Approved
|
[1]
|
| Structure |
|
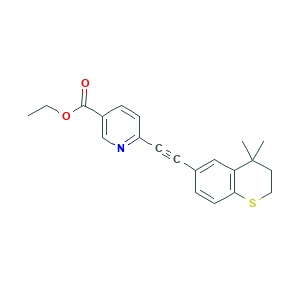 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
351.5 |
Topological Polar Surface Area |
64.5 |
| Heavy Atom Count |
25 |
Rotatable Bond Count |
5 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 5381
- PubChem SID
-
582921
; 4405151
; 7848195
; 8153307
; 12014289
; 14754274
; 29224433
; 46508992
; 50031802
; 50112778
; 53789348
; 56352905
; 57322745
; 68530738
; 79123421
; 85246194
; 92719256
; 93166451
; 103629338
; 104113517
; 104253218
; 104253308
; 104309077
; 124757343
; 125164147
; 125338838
; 126620953
; 126658167
; 126670670
; 127300883
; 127300884
; 127300885
; 127300886
; 127300887
; 127300888
; 127300889
; 127300890
; 127300891
; 127300892
; 127300893
; 127300894
; 127300895
; 127300896
; 127300897
; 128806208
; 134337757
; 135017550
; 135692178
; 136376512
; 136946544
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0BM5G
- Formula
- C21H21NO2S
- Canonical SMILES
- CCOC(=O)C1=CN=C(C=C1)C#CC2=CC3=C(C=C2)SCCC3(C)C
- InChI
- 1S/C21H21NO2S/c1-4-24-20(23)16-7-9-17(22-14-16)8-5-15-6-10-19-18(13-15)21(2,3)11-12-25-19/h6-7,9-10,13-14H,4,11-12H2,1-3H3
- InChIKey
- OGQICQVSFDPSEI-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


