| General Information of Drug (ID:
DR1558) |
| Drug Name |
Terfenadine
|
| Prodrug Info |
Terfenadine is the prodrug of Fexofenadine
|
| Synonyms |
Teldane; Teldanex; Terdin; Terfen; Terfenadina; Terfenadina [INN-Spanish]; Terfenadinum; Terfenadinum [INN-Latin]; Terfex; Ternadin; Triludan; Aldaban; Allerplus; Cyater; GUGOEEXESWIERI-UHFFFAOYSA-N; MDL 9918; MDL-9918; RMI 9918; RMI-9918; Seldane; terfenadine; 50679-08-8; BRN 5857899; C32H41NO2; CHEBI:9453; CHEMBL17157; EINECS 256-710-8; HSDB 6508; MFCD00079622; MLS000028499; alpha-(p-tert-Butylphenyl)-4-(hydroxydiphenylmethyl)-1-piperidinebutanol; alpha-[4-(1,1-Dimethylethyl)phenyl]-4-(hydroxydiphenylmethyl)-1-piperidinebutanol
|
| Indication |
Allergy
[ICD11: 4A80]
|
Phase 4
|
[1]
|
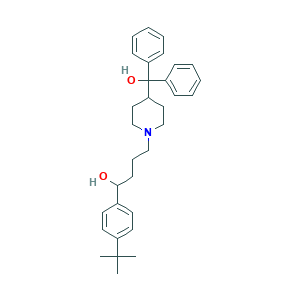
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
471.7 |
Topological Polar Surface Area |
43.7 |
| Heavy Atom Count |
35 |
Rotatable Bond Count |
9 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 5405
- PubChem SID
-
9666
; 511394
; 855647
; 3154381
; 5639782
; 7847587
; 7980761
; 8150039
; 8153324
; 10321465
; 10524514
; 11336186
; 11361425
; 11427139
; 11462397
; 11466166
; 11467286
; 11485891
; 12013836
; 14907543
; 17405767
; 24277779
; 26752308
; 29224457
; 46507007
; 47662382
; 47662383
; 47736580
; 47810839
; 47810840
; 48035221
; 48110535
; 48416601
; 49698808
; 49703451
; 50015354
; 50105228
; 50105229
; 53778319
; 53787217
; 56422373
; 57322760
; 79821290
; 85089724
; 85209594
; 85231279
; 85787897
; 90341346
; 92125182
; 92303330
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D08SOF
- Formula
- C32H41NO2
- Canonical SMILES
- CC(C)(C)C1=CC=C(C=C1)C(CCCN2CCC(CC2)C(C3=CC=CC=C3)(C4=CC=CC=C4)O)O
- InChI
- 1S/C32H41NO2/c1-31(2,3)26-18-16-25(17-19-26)30(34)15-10-22-33-23-20-29(21-24-33)32(35,27-11-6-4-7-12-27)28-13-8-5-9-14-28/h4-9,11-14,16-19,29-30,34-35H,10,15,20-24H2,1-3H3
- InChIKey
- GUGOEEXESWIERI-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


