| General Information of Drug (ID:
DR1581) |
| Drug Name |
Thioridazine
|
| Synonyms |
Thioridazin; Thioridazine prolongatum; Thioridazine, prolongatum; Thioridazinum; Thioridazinum [INN-Latin]; Tioridazin; Tioridazina; Tioridazina [INN-Spanish]; Mallorol; Malloryl; Meleril; Mellaril; Mellaril-S; Mellarit; Mellerets; Mellerette; Melleretten; Melleril; Melleril (liquid); Melleryl; Metlaril; Orsanil; Ridazine; Sonapax; dl-Thioridazine; thioridazine; (+-)-Thioridazine; 10-(2-(1-Methylpiperidin-2-yl)ethyl)-2-(methylthio)-10H-phenothiazine; 2-Methylmercapto-10-(2-(N-methyl-2-piperidyl)ethyl)phenothiazine; 50-52-2; TP-21
|
| Indication |
Schizophrenia
[ICD11: 6A20]
|
Approved
|
[1]
|
| Structure |
|
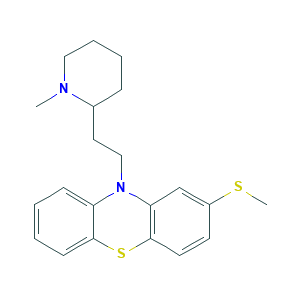 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
370.6 |
Topological Polar Surface Area |
57.1 |
| Heavy Atom Count |
25 |
Rotatable Bond Count |
4 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 5452
- PubChem SID
-
616470
; 5646742
; 7847439
; 7980776
; 8153353
; 10524427
; 11335241
; 11360480
; 11363960
; 11364721
; 11366522
; 11367283
; 11369084
; 11369845
; 11371630
; 11372886
; 11373930
; 11375445
; 11377246
; 11378009
; 11461452
; 11466106
; 11467226
; 11485219
; 11485952
; 11489148
; 11490401
; 11492171
; 11494880
; 12013415
; 14804036
; 26752322
; 29224497
; 46509070
; 47216619
; 47216620
; 47290971
; 47440079
; 47515153
; 47515154
; 47588829
; 47810593
; 48110292
; 48334314
; 48334315
; 48416615
; 49658718
; 49698892
; 50006598
; 50105272
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0U1OE
- Formula
- C21H26N2S2
- Canonical SMILES
- CN1CCCCC1CCN2C3=CC=CC=C3SC4=C2C=C(C=C4)SC
- InChI
- 1S/C21H26N2S2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(24-2)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3
- InChIKey
- KLBQZWRITKRQQV-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


