| Synonyms |
Ticlopidina; Ticlopidina [INN-Spanish]; PCR 5332; PHWBOXQYWZNQIN-UHFFFAOYSA-N; Ticlid; Ticlopidine [INN:BAN]; Ticlopidinum; Ticlopidinum [INN-Latin]; ticlopidine; 5-((2-Chlorophenyl)methyl)-4,5,6,7-tetrahydrothieno(3,2-c)pyridine; 5-(2-Chlorobenzyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine; 5-[(2-chlorophenyl)methyl]-4,5,6,7-tetrahydrothieno[3,2-c]pyridine; 5-[(2-chlorophenyl)methyl]-4H,5H,6H,7H-thieno[3,2-c]pyridine; 55142-85-3; BRN 1216802; C14H14ClNS; CHEBI:9588; EINECS 259-498-5; UNII-OM90ZUW7M1; OM90ZUW7M1
|
| Cross-matching ID |
- PubChem CID
- 5472
- PubChem SID
-
9349
; 5650061
; 7980793
; 8153361
; 10526772
; 11112786
; 11466075
; 11467195
; 11485830
; 15197138
; 26751617
; 29224517
; 46504438
; 47291227
; 47440368
; 47589090
; 48110545
; 48416628
; 49698319
; 50053360
; 50085878
; 50100522
; 50139240
; 50661300
; 57288855
; 57322795
; 61110231
; 85209800
; 85787491
; 90945105
; 92308854
; 92714062
; 93167187
; 93625532
; 96025278
; 99309316
; 103219164
; 104171328
; 104309332
; 117785149
; 124882622
; 124882623
; 124882624
; 124882625
; 124882626
; 124882627
; 125203923
; 126629679
; 126666024
; 128918298
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D05LBU
- Formula
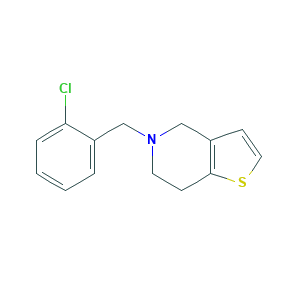
- C14H14ClNS
- Canonical SMILES
- C1CN(CC2=C1SC=C2)CC3=CC=CC=C3Cl
- InChI
- 1S/C14H14ClNS/c15-13-4-2-1-3-11(13)9-16-7-5-14-12(10-16)6-8-17-14/h1-4,6,8H,5,7,9-10H2
- InChIKey
- PHWBOXQYWZNQIN-UHFFFAOYSA-N
|