| Synonyms |
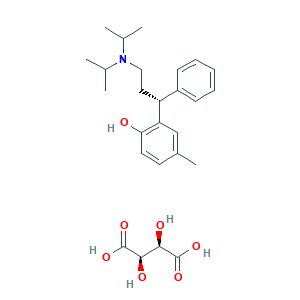
Tolterodine (tartrate); Tolterodine tartrate (Detrol LA); Tolterodine tartrate [USAN]; UNII-5T619TQR3R; Tolterodina; Tolterodinum; UNII-WHE7A56U7K; Unidet; WHE7A56U7K; tolterodine; (+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl)-p-cresol; (+)-Tolterodine; (R)-(+)-Tolterodine; 124937-51-5; 2-[(1R)-3-(diisopropylamino)-1-phenylpropyl]-4-methylphenol; CHEBI:9622; CHEMBL1382; DSSTox_CID_3687; DSSTox_GSID_23687; DSSTox_RID_77147; Kabi 2234; NCGC00159519-02; PNU 200583; Q-200223; S-(-)-Tolterodine; (+)-(R)-2-(I-(2-(Diisopropylamino)ethyl)benzyl)-p-cresol L-tartrate (1:1) (salt); (R)-2-(3-(Diisopropylamino)-1-phenylpropyl)-4-methylphenol (2R,3R)-2,3-dihydroxysuccinate; (R)-2-(3-Diisopropylamino-1-phenyl-propyl)-p-cresol L-tartrate; 124937-52-6; 5T619TQR3R; C26H37NO7; Detrol LA; PNU 200583E; PNU-200583E
|
| Cross-matching ID |
- PubChem CID
- 443878
- CAS Number
-
- TTD Drug ID
- D0BZ7W
- Formula
- C26H37NO7
- Canonical SMILES
- CC1=CC(=C(C=C1)O)C(CCN(C(C)C)C(C)C)C2=CC=CC=C2.C(C(C(=O)O)O)(C(=O)O)O
- InChI
- 1S/C22H31NO.C4H6O6/c1-16(2)23(17(3)4)14-13-20(19-9-7-6-8-10-19)21-15-18(5)11-12-22(21)24;5-1(3(7)8)2(6)4(9)10/h6-12,15-17,20,24H,13-14H2,1-5H3;1-2,5-6H,(H,7,8)(H,9,10)/t20-;1-,2-/m11/s1
- InChIKey
- TWHNMSJGYKMTRB-KXYUELECSA-N
|