| General Information of Drug (ID:
DR1631) |
| Drug Name |
Trazodone hydrochloride
|
| Synonyms |
Thombran; Tombran; Trazodone (hydrochloride); Trazodone Hcl; Trazodone hydrochloride; Trazodone hydrochloride (Desyrel); Tritico; Triticum; Trazalon; Trazodil; Trazodon; Trazodona; Trazodona [INN-Spanish]; Trazodona [Spanish]; Trazodone [INN:BAN]; Trazodonum; Trazodonum [INN-Latin]; Trazodonum [Latin]; Trazonil; Trialodine; UNII-YBK48BXK30; YBK48BXK30; s-Triazolo(4,3-a)pyridin-3(2H)-one, 2-(3-(4-(m-chlorophenyl)-1-piperazinyl)propyl)-; Trazodone; 19794-93-5; BRN 0628010; Beneficat; C19H22ClN5O; CHEBI:9654; CHEMBL621; Desirel; EINECS 243-317-1; PHLBKPHSAVXXEF-UHFFFAOYSA-N; Sideril; UNII-6E8ZO8LRNM; 2-(3-(4-(m-Chlorophenyl)-1-piperazinyl)propyl)-s-triazolo(4,3-a)pyridin-3(2H)-one monohydrochloride; 25332-39-2; 6E8ZO8LRNM; AF 1161; AF-1161; Apo-Trazodone; Azona; Bimaran; CHEBI:9655; DSSTox_RID_80361; Devidon; EINECS 246-855-5; HSDB 7048; KB-831; MFCD00079603; MLS000069698; NSC 292811; Pragmazone; SMR000058520
|
| Indication |
Depression
[ICD11: 6A71]
|
Approved
|
[1]
|
| Structure |
|
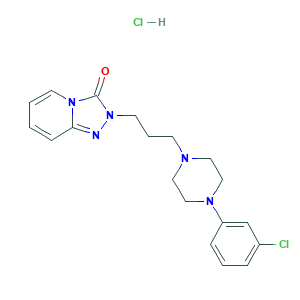 |
|
3D MOL is unavailable
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
408.3 |
Topological Polar Surface Area |
42.4 |
| Heavy Atom Count |
27 |
Rotatable Bond Count |
5 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 62935
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D00USF
- Formula
- C19H23Cl2N5O
- Canonical SMILES
- C1CN(CCN1CCCN2C(=O)N3C=CC=CC3=N2)C4=CC(=CC=C4)Cl.Cl
- InChI
- 1S/C19H22ClN5O.ClH/c20-16-5-3-6-17(15-16)23-13-11-22(12-14-23)8-4-10-25-19(26)24-9-2-1-7-18(24)21-25;/h1-3,5-7,9,15H,4,8,10-14H2;1H
- InChIKey
- OHHDIOKRWWOXMT-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


