Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1637) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Triazolam
|
|||||
| Synonyms |
Triazolamum; Triazolamum [INN-Latin]; Clorazolam; Halcion; JOFWLTCLBGQGBO-UHFFFAOYSA-N; Novidorm; Novodorm; Songar; U 33030; U-33,030; U-33030; triazolam; 28911-01-5; 8-Chloro-6-(2-chlorophenyl)-1-methyl-4H-(1,2,4)triazolo(4,3-a)(1,4)benzodiazepine; 8-Chloro-6-(2-chlorophenyl)-1-methyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine; 8-Chloro-6-(o-chlorophenyl)-1-methyl-4H-s-triazolo(4,3-a)(1,4)benzodiazepine; BRN 1226643; C17H12Cl2N4; CCRIS 1932; CHEBI:9674; CHEMBL646; DEA No. 2887; EINECS 249-307-3; HSDB 6759; UNII-1HM943223R
|
|||||
| Indication | Insomnia [ICD11: 7A00] | Approved | [1] | |||
| Structure |
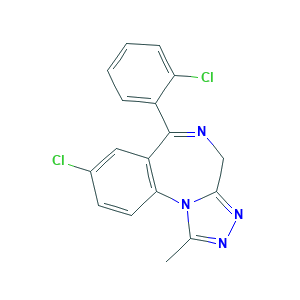 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 343.2 | Topological Polar Surface Area | 43.1 | ||
| Heavy Atom Count | 23 | Rotatable Bond Count | 1 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 3 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

