Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1647) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Trimethadione
|
|||||
| Synonyms |
Tioxanona; Tredione; Tricione; Tridilona; Tridion; Tridione; Tridone; Trilidona; Trimedal; Trimedone; Trimetadione; Trimetadione [DCIT]; Trimethadion; Trimethadionum; Trimethdione; Trimethin; Trimethinum; Trimetin; Trioksal; Trioxanona; Triozanona; Tromedone; Troxidone; trimethadione; 127-48-0; 2,4-OXAZOLIDINEDIONE, 3,5,5-TRIMETHYL-; Convenixa; Convexina; Epidione; Epidone; Epixal; Etydion; Minoaleuiatin; Minoaleviatin; Petidion; Petidon; Petilep; Petimalin; Pitmal; Ptimal; 3,5,5-Trimethyloxazolidine-2,4-dione; Absentol; Absetil; Edion
|
|||||
| Indication | Epilepsy [ICD11: 8A60] | Approved | [1] | |||
| Structure |
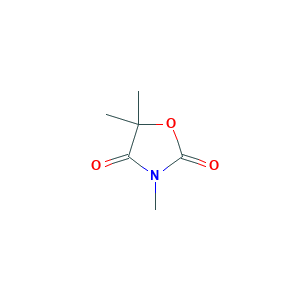 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 143.14 | Topological Polar Surface Area | 46.6 | ||
| Heavy Atom Count | 10 | Rotatable Bond Count | 0 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 3 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

