| Cross-matching ID |
- PubChem CID
- 60857
- PubChem SID
-
9427
; 7847481
; 7980917
; 8187100
; 11484392
; 11488544
; 11528684
; 12014671
; 14897880
; 14922369
; 26612872
; 26680025
; 26719749
; 43118195
; 46386880
; 46386934
; 46506452
; 46530532
; 49681573
; 50730852
; 57314155
; 91011737
; 92124588
; 92308124
; 92308640
; 93166198
; 96099961
; 103346351
; 103941674
; 104179250
; 104253285
; 104321827
; 117664449
; 118855343
; 124636835
; 124757406
; 124801240
; 125164210
; 126656630
; 126667001
; 129386325
; 135017989
; 135651366
; 135692210
; 135693782
; 136974858
; 137171693
; 142742126
; 144076376
; 144205008
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0NG7O
- Formula
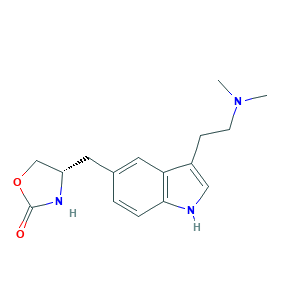
- C16H21N3O2
- Canonical SMILES
- CN(C)CCC1=CNC2=C1C=C(C=C2)CC3COC(=O)N3
- InChI
- 1S/C16H21N3O2/c1-19(2)6-5-12-9-17-15-4-3-11(8-14(12)15)7-13-10-21-16(20)18-13/h3-4,8-9,13,17H,5-7,10H2,1-2H3,(H,18,20)/t13-/m0/s1
- InChIKey
- ULSDMUVEXKOYBU-ZDUSSCGKSA-N
|