| Synonyms |
Zonegran; Zonisamida; Zonisamida [Spanish]; Zonisamidum; Zonisamidum [Latin]; Exceglan; Excegram; Excegran; PD 110843; PD-110843; zonisamide; 1,2-Benzisoxazole-3-methanesulfonamide; 1,2-benzoxazol-3-ylmethanesulfonamide; 1-(1,2-Benzoxazol-3-Yl)methanesulfonamide; 1-(1,2-benzisoxazol-3-yl)methanesulfonamide; 3-(Sulfamoylmethyl)-1,2-benzisoxazole; 68291-97-4; AD 810; AD-810; BRN 1077076; Benzo[d]isoxazol-3-yl-methanesulfonamide; C8H8N2O3S; CI 912; CI-912; HSDB 7293; SPR_2; UNII-459384H98V; benzo[d]isoxazol-3-ylmethanesulfonamide
|
| Cross-matching ID |
- PubChem CID
- 5734
- PubChem SID
-
9707
; 5375171
; 7847604
; 8153515
; 11528623
; 12012838
; 12016186
; 15220241
; 29215225
; 29224771
; 46505278
; 46511458
; 49666067
; 50086000
; 50109845
; 57322921
; 74524371
; 81093137
; 85174242
; 85788213
; 87322638
; 92308963
; 92714085
; 92729687
; 93166866
; 93167201
; 99437155
; 103147645
; 103200464
; 103911541
; 104170135
; 104310103
; 105859475
; 117532844
; 117843348
; 118048699
; 121362594
; 124757254
; 124801297
; 125164058
; 125324718
; 125356086
; 126629668
; 126655670
; 126670159
; 129345232
; 130010354
; 131323538
; 131377323
; 134337793
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D09ZIS
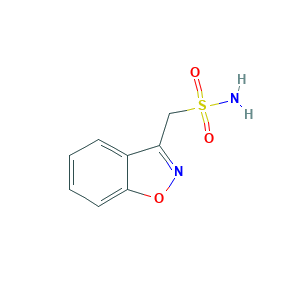
- Formula
- C8H8N2O3S
- Canonical SMILES
- C1=CC=C2C(=C1)C(=NO2)CS(=O)(=O)N
- InChI
- 1S/C8H8N2O3S/c9-14(11,12)5-7-6-3-1-2-4-8(6)13-10-7/h1-4H,5H2,(H2,9,11,12)
- InChIKey
- UBQNRHZMVUUOMG-UHFFFAOYSA-N
|