| Synonyms |
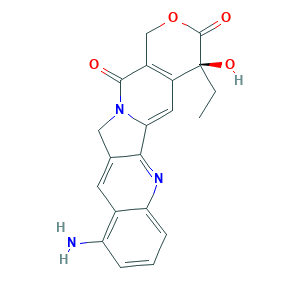
Camptothecin, 9-amino-; (4s)-10-amino-4-ethyl-4-hydroxy-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione; (S)-10-Amino-4-ethyl-4-hydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione; 5MB77ICE2Q; 9-AMINO-20(S)-CAMPTOTHECIN; 9-Amino-20-(S)-camptothecin; 9-Amino-camptothecin; 9-Amino-cpt; 9-Aminocamptothecin; 91421-43-1; AK-88555; CHEBI:80755; CHEMBL274070; NSC 603071; NSC-603071; NSC603071; UNII-5MB77ICE2Q
|
| Cross-matching ID |
- PubChem CID
- 72402
- PubChem SID
-
483848
; 600881
; 8195177
; 11408968
; 12014702
; 14876953
; 14876954
; 26725257
; 43128571
; 50028358
; 57318699
; 77695393
; 87560557
; 96023346
; 99373552
; 99432308
; 103073229
; 103082936
; 103170467
; 103910998
; 103922005
; 104352855
; 117586313
; 119525751
; 124796287
; 124953919
; 127347071
; 127903358
; 135030378
; 137138142
; 142371086
; 152036014
; 152227690
; 160809182
; 162179182
; 162937855
; 163374696
; 164758081
; 170559265
; 172860100
; 174531450
; 178115205
; 179151428
; 184538696
; 188900245
; 198973567
; 223445740
; 223603491
; 225031198
; 226414396
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D07UHX
- Formula
- C20H17N3O4
- Canonical SMILES
- CCC1(C2=C(COC1=O)C(=O)N3CC4=CC5=C(C=CC=C5N=C4C3=C2)N)O
- InChI
- 1S/C20H17N3O4/c1-2-20(26)13-7-16-17-10(6-11-14(21)4-3-5-15(11)22-17)8-23(16)18(24)12(13)9-27-19(20)25/h3-7,26H,2,8-9,21H2,1H3/t20-/m0/s1
- InChIKey
- FUXVKZWTXQUGMW-FQEVSTJZSA-N
|