Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1941) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Curcumin
|
|||||
| Synonyms |
Curcuma; Curcuma oil; Curcumin I; Curcumine; Diferaloylmethane; Diferuloylmethane; Gelbwurz; Golden seal; Haldar; Hydrastis; Indian saffron; Indian turmeric; Kacha haldi; Kurkumin [Czech]; Merita earth; Natural yellow 3; Oils, curcuma; Orange Root; Safran d'Inde; Souchet; Terra Merita; Tumeric yellow; Turmeric; Turmeric extract; Turmeric oil; Turmeric yellow; Yellow Ginger; Yellow Root; Yellow puccoon; Yo-Kin; curcumin; (1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione; 458-37-7; C.I. Natural Yellow 3; Haidr; Halad; Halud
|
|||||
| Indication | Stomach cancer [ICD11: 2B72] | Phase 3 | [1] | |||
| Structure |
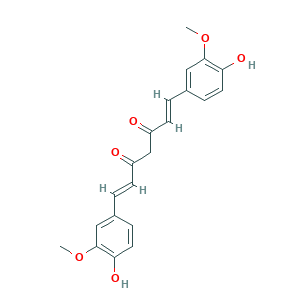 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 368.4 | Topological Polar Surface Area | 93.1 | ||
| Heavy Atom Count | 27 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 6 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Enzyme Kinetic Data of This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | Nanocurcumin: a promising therapeutic advancement over native curcumin. Crit Rev Ther Drug Carrier Syst. 2013;30(4):331-68. | |||||
| 2 | Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc Natl Acad Sci U S A. 2011 Apr 19;108(16):6615-20. | |||||
| 3 | Differential and special properties of the major human UGT1-encoded gastrointestinal UDP-glucuronosyltransferases enhance potential to control chemical uptake. J Biol Chem. 2004 Jan 9;279(2):1429-41. | |||||
| 4 | Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr Med Chem. 2009;16(27):3480-675. | |||||
| 5 | Curcumin and Weight Loss: Does It Work? | |||||
| 6 | Curcumin, Gut Microbiota, and Neuroprotection | |||||
| 7 | Biological and pharmacological effects of hexahydrocurcumin, a metabolite of curcumin | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

