| Synonyms |
Acetyl CoA; Coenzyme A, S-acetate; S-Acetyl coenzyme A; S-Acetylcoenzyme A; S-acetyl-CoA; S-acetyl-coenzyme A; acetyl coenzyme-A; acetyl-CoA; acetyl-S-CoA; acetylCoA; acetylcoenzyme-A; 3'-phosphoadenosine 5'-(3-{(3R)-4-[(3-{[2-(acetylsulfanyl)ethyl]amino}-3-oxopropyl)amino]-3-hydroxy-2,2-dimethyl-4-oxobutyl} dihydrogen diphosphate); ACETYL COENZYME A; 72-89-9; 76Q83YLO3O; AcCoA; UNII-76Q83YLO3O; ac-CoA; ac-S-CoA
|
| Cross-matching ID |
- PubChem CID
- 444493
- PubChem SID
-
1779
; 3326
; 585640
; 820704
; 822294
; 823218
; 824919
; 826846
; 829086
; 829103
; 829806
; 831238
; 831240
; 834102
; 837311
; 841347
; 855085
; 7885691
; 8027383
; 8028055
; 8028566
; 10299538
; 10317655
; 10318175
; 10318247
; 10318521
; 11110405
; 11110459
; 14710306
; 14710334
; 14710480
; 17404244
; 17422545
; 17424997
; 24277059
; 24277587
; 24424435
; 24424471
; 24778751
; 24778980
; 26703508
; 26708546
; 26716778
; 26718485
; 26718888
; 29196119
; 29211946
; 36887496
; 49742999
; 50126532
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D01AWE
- Formula
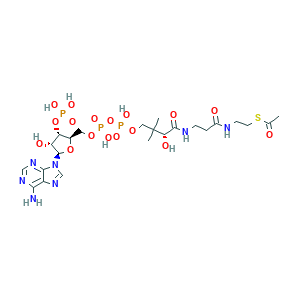
- C23H38N7O17P3S
- Canonical SMILES
- CC(=O)SCCNC(=O)CCNC(=O)C(C(C)(C)COP(=O)(O)OP(=O)(O)OCC1C(C(C(O1)N2C=NC3=C(N=CN=C32)N)O)OP(=O)(O)O)O
- InChI
- 1S/C23H38N7O17P3S/c1-12(31)51-7-6-25-14(32)4-5-26-21(35)18(34)23(2,3)9-44-50(41,42)47-49(39,40)43-8-13-17(46-48(36,37)38)16(33)22(45-13)30-11-29-15-19(24)27-10-28-20(15)30/h10-11,13,16-18,22,33-34H,4-9H2,1-3H3,(H,25,32)(H,26,35)(H,39,40)(H,41,42)(H2,24,27,28)(H2,36,37,38)/t13-,16-,17-,18+,22-/m1/s1
- InChIKey
- ZSLZBFCDCINBPY-ZSJPKINUSA-N
|