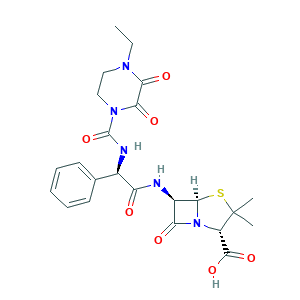
| Synonyms |
Peperacillin; Peracin; Peracin (TN); Piperacillin (INN); Piperacillin (anhydrous); Piperacillin Monosodium Salt; Piperacillin anhydrous; Pipercillin; Pipracil; Pipracil, Piper; Pipril; PIPERACILLIN SODIUM; T-1220; Zobactin (TN); (2S,5R,6R)-6-[[(2R)-2-[(4-ethyl-2,3-dioxopiperazine-1-carbonyl)amino]-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (2S,5R,6R)-6-{[(2R)-2-{[(4-ethyl-2,3-dioxopiperazin-1-yl)carbonyl]amino}-2-phenylacetyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (2S-(2alpha,5alpha,6beta(S*)))-6-(((((4-Ethyl-2,3-dioxopiperazin-1-yl)carbonyl)amino)phenylacetyl)amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid; 4-ethyl-2,3-dioxopiperazine carbonyl ampicillin; 6-(D-(-)-alpha-(4-Ethyl-2,3-dioxo-1-piperazinecarboxamido)phenylacetamido)penicillanicacid; 6beta-{(2R)-2-[(4-ethyl-2,3-dioxopiperazin-1-yl)carboxamido]-2-phenylacetamido}-2,2-dimethylpenam-3alpha-carboxylic acid; Cl-227193; PIPC
|
| Cross-matching ID |
- PubChem CID
- 43672
- PubChem SID
-
854275
; 7835955
; 7980328
; 8178297
; 11466783
; 11467903
; 11486445
; 14860547
; 14885009
; 34709064
; 46504757
; 47216691
; 47736382
; 47959647
; 48035016
; 48416441
; 50050723
; 50953976
; 53786998
; 57312854
; 58106750
; 85788489
; 92309155
; 93166325
; 96025066
; 104098277
; 104234163
; 104342936
; 117541876
; 124766128
; 126685780
; 127341075
; 127341076
; 127341077
; 131465223
; 134338409
; 135006048
; 135951164
; 137248591
; 140093228
; 142043554
; 163403896
; 163846739
; 164232663
; 164814522
; 175266788
; 175442177
; 176484439
; 179116854
; 185975394
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D04ZAH
- Formula
- C23H27N5O7S
- Canonical SMILES
- CCN1CCN(C(=O)C1=O)C(=O)NC(C2=CC=CC=C2)C(=O)NC3C4N(C3=O)C(C(S4)(C)C)C(=O)O
- InChI
- 1S/C23H27N5O7S/c1-4-26-10-11-27(19(32)18(26)31)22(35)25-13(12-8-6-5-7-9-12)16(29)24-14-17(30)28-15(21(33)34)23(2,3)36-20(14)28/h5-9,13-15,20H,4,10-11H2,1-3H3,(H,24,29)(H,25,35)(H,33,34)/t13-,14-,15+,20-/m1/s1
- InChIKey
- IVBHGBMCVLDMKU-GXNBUGAJSA-N
|