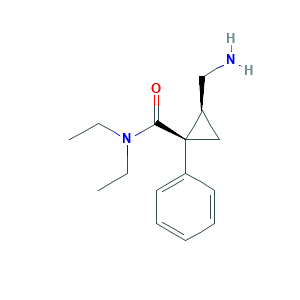
| Synonyms |
Midalcipran; Milnace; Milnacipran; Milnacipran (INN); Milnacipran [INN]; Milnacipranum; Milnacipranum [Latin]; (+-)-Milnacipran; Dalcipran; Dextromilnacipran; ES1O38J3C4; (+-)-cis-2-(Aminomethyl)-N,N-diethyl-1-phenylcyclopropanecarboxamide; (1R,2S)-2-(aminomethyl)-N,N-diethyl-1-phenylcyclopropane-1-carboxamide; (1R,2S)-2-(aminomethyl)-N,N-diethyl-1-phenylcyclopropanecarboxamide; 92623-85-3; Cyclopropanecarboxamide, 2-(aminomethyl)-N,N-diethyl-1-phenyl-, cis-(+-)-; F 2207; F2207; Ixel; UNII-ES1O38J3C4
|
| Cross-matching ID |
- PubChem CID
- 65833
- ChEBI ID
-
- CAS Number
-
- Formula
- C15H22N2O
- Canonical SMILES
- CCN(CC)C(=O)C1(CC1CN)C2=CC=CC=C2
- InChI
- 1S/C15H22N2O/c1-3-17(4-2)14(18)15(10-13(15)11-16)12-8-6-5-7-9-12/h5-9,13H,3-4,10-11,16H2,1-2H3/t13-,15+/m1/s1
- InChIKey
- GJJFMKBJSRMPLA-HIFRSBDPSA-N
|