| Synonyms |
Alitretinoin; 9-CIS-RETINOIC ACID; Panretin; 9-cis-Tretinoin; Panrexin; 9-cis Retinoic Acid; 5300/3/8; Panretyn; Panretin Gel; (9cis)-retinoic acid; Toctino; 9(Z)-Retinoic acid; Panretin (TN); Alitretinoin (USAN); Alitretinoin [USAN]; Retinoic acid, 9-cis-; ALRT-1057; trans-Vitamin a acid; ALRT 1057; BAL-4079; LGD-1057; CHEMBL705; UNII-1UA8E65KDZ; AGN 192013; AGN-192013; LGD 100057; NSC-659772; 1UA8E65KDZ; Atragen; Retinova; LG-100057; ALRT1057; CCRIS 7098; CHEBI:50648; HSDB 7186; LGD1057; SHGAZHPCJJPHSC-ZVCIMWCZSA-N; Retinoic acid, cis-9,trans-13-; Tretinoin (TN); (7E,9Z,11E,13E)-retinoic acid; (2E,4E,6Z,8E)-3,7-Dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoic acid; NSC 659772; 9-cis-RA; Retin A (TN); LG100057; trans-Retinoate; beta-Retinoate; 9 Cis Retinoic Acid; alitretinoina; alitretinoine
|
| Cross-matching ID |
- PubChem CID
- 449171
- PubChem SID
-
509159
; 584816
; 838980
; 3718107
; 7978614
; 8026464
; 10300319
; 11528315
; 12014910
; 14898408
; 17396485
; 17396973
; 17486479
; 22391507
; 24702085
; 24899373
; 25622044
; 25641192
; 26681478
; 26718599
; 26755227
; 26755228
; 26755229
; 26755230
; 36890827
; 46507356
; 47811003
; 47885624
; 47885625
; 50087219
; 50110876
; 50110877
; 50110878
; 53790163
; 53801213
; 56310968
; 56313931
; 56313962
; 57288659
; 57404908
; 57571034
; 75174291
; 85240518
; 85279329
; 85788016
; 87219231
; 90451653
; 92309911
; 99300825
; 99302343
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0G3PI
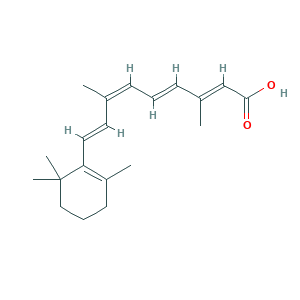
- Formula
- C20H28O2
- Canonical SMILES
- CC1=C(C(CCC1)(C)C)C=CC(=CC=CC(=CC(=O)O)C)C
- InChI
- 1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+
- InChIKey
- SHGAZHPCJJPHSC-ZVCIMWCZSA-N
|