Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR5347) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
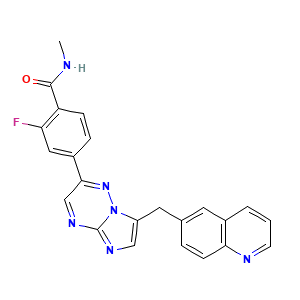
Capmatinib
|
|||||
| Synonyms |
1029712-80-8; INCB28060; INC-280; INC280; UNII-TY34L4F9OZ; 2-fluoro-N-methyl-4-(7-(quinolin-6-ylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl)benzamide; INC28060; INCB-28060; INCB28060(Capmatinib); NVP-INC280; TY34L4F9OZ; Capmatinib (INCB28060); INCB 28060; 2-Fluoro-N-methyl-4-[7-[(quinolin-6-yl)methyl]imidazo[1,2-b]-[1,2,4]triazin-2-yl]benzamide; BenzaMide, 2-fluoro-N-Methyl-4-[7-(6-quinolinylMethyl)iMidazo[1,2-b][1,2,4]triazin-2-yl]-; C23H17FN6O
|
|||||
| Indication | Lung cancer [ICD11: 2C25] | Approved | [1] | |||
| Hepatocellular carcinoma [ICD11: ICD11: 2C12] | Phase 2 | [2] | ||||
| Brain cancer [ICD11: ICD11: 2A00] | Phase 1 | [3] | ||||
| Structure |
 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 412.4 | Topological Polar Surface Area | 85.1 | ||
| Heavy Atom Count | 31 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 6 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2020 | |||||
| 2 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800031741) | |||||
| 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| 4 | Absorption, Distribution, Metabolism, and Excretion of Capmatinib (INC280) in Healthy Male Volunteers and In Vitro Aldehyde Oxidase Phenotyping of the Major Metabolite | |||||
| 5 | Pharmacokinetics of capmatinib in participants with hepatic impairment: A phase 1, open-label, single-dose, parallel-group study | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

