| General Information of This Metabolic Reaction (MR) (ID:
MR001737) |
| Formula |
|
| Reactant |
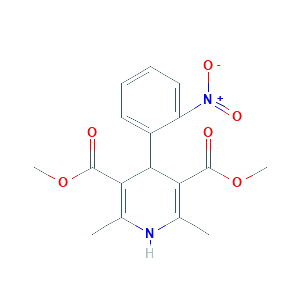
Nifedipine |
Product |
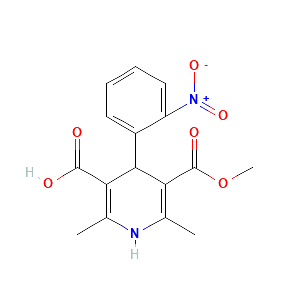
2,6-dimethyl-4-(2-nitrophenyl)-5-methoxycarbonyl-pyridine-3-carboxylic acid |
|
Reactant Info
|
Product Info
|
|
Metabolic Enzyme
|
Cytochrome P450 3A4 (CYP3A4)
|
DME Info
|
|
Cytochrome P450 3A5 (CYP3A5)
|
DME Info
|
|
|
|
|
|
|
|
| Other MR(s) Related to The Reactant of This MR |
|
Other MR(s) That Metabolize The Reactant of This MR
|
|
|
| Other MR(s) Related to The Product of This MR |
|
Other MR(s) That Produce The Product of This MR
|
|
|
|
Other MR(s) That Metabolize The Produtc of This MR
|
|
|
| References |
| 1 |
The first pass metabolism of nifedipine in man Br J Clin Pharmacol. 1984 Dec;18(6):951-4. doi: 10.1111/j.1365-2125.1984.tb02569.x.
|
| 2 |
Pharmacokinetics and metabolism of nifedipine Hypertension. 1983 Jul-Aug;5(4 Pt 2):II18-24. doi: 10.1161/01.hyp.5.4_pt_2.ii18.
|
| 3 |
Clinical pharmacokinetics of nisoldipine coat-core Clin Pharmacokinet. 1998 Sep;35(3):191-208. doi: 10.2165/00003088-199835030-00003.
|
| 4 |
CYP3A5 genotype did not impact on nifedipine disposition in healthy volunteers Pharmacogenomics J. 2004;4(1):34-9. doi: 10.1038/sj.tpj.6500218.
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.