| References |
| 1 |
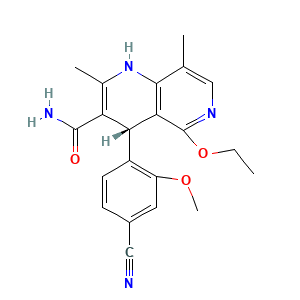
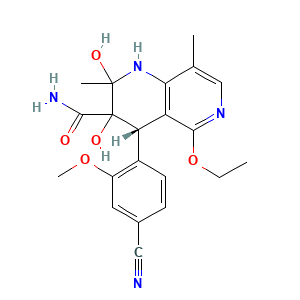
Biotransformation of Finerenone, a Novel Nonsteroidal Mineralocorticoid Receptor Antagonist, in Dogs, Rats, and Humans, In Vivo and In Vitro
|
| 2 |
Aldosterone and Mineralocorticoid Receptor Signaling as Determinants of Cardiovascular and Renal Injury: From Hans Selye to the Present
|
| 3 |
Results From Drug-Drug Interaction Studies In Vitro and In Vivo Investigating the Inhibitory Effect of Finerenone on the Drug Transporters BCRP, OATP1B1, and OATP1B3
|
| 4 |
DrugBank(Pharmacology-Metabolism):Finerenone
|