| References |
| 1 |
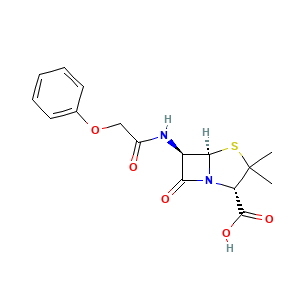
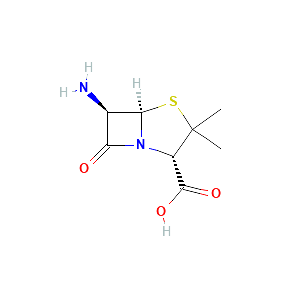
Enzyme-catalyzed biodegradation of penicillin fermentation residues by -lactamase OtLac from Ochrobactrum tritici
|
| 2 |
Isolation and Characterization of Ochrobactrum tritici for Penicillin V Potassium Degradation
|
| 3 |
Sex- and species-related nephropathy of 6-(1-aminocyclohexanecarboxamido)penicillanic acid (cyclacillin) and its relationship to the metabolic disposition of the drug
|
| 4 |
Metabolism of penicillins to penicilloic acids and 6-aminopenicillanic acid in man and its significance in assessing penicillin absorption
|
| 5 |
Ligand-induced conformational change in penicillin acylase
|
| 6 |
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD:American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 1760
|