Detail Information of Xenobiotics
| General Information of Xenobiotics (ID: XEO00334) | ||||||
|---|---|---|---|---|---|---|
| Xenobiotics Name |
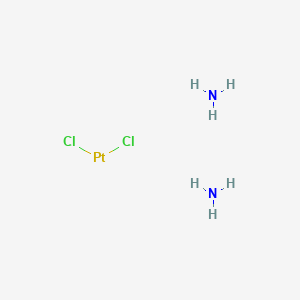
Cisplatin
|
|||||
| Xenobiotics Type |
Pharmaceutical Agent(s)
|
|||||
| Classification |
Approved/Marketed Drug
|
|||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=5702198"></iframe>
|
 |
||||
| 3D MOL | 2D MOL | |||||
| PubChem CID | ||||||
| DME(s) Modulated by This Xenobiotics | ||||||
| DME(s) Inhibited by This Xenobiotics | ||||||
| Aldo-keto reductase 1B1 (AKR1B1) | DME Info | Homo sapiens | [1] | |||
| Aldo-keto reductase 1C2 (AKR1C2) | DME Info | Homo sapiens | [2] | |||
| Aldehyde dehydrogenase 1 (ALDH1) | DME Info | Homo sapiens | [3] | |||
| Aldehyde dehydrogenase 7 (ALDH7) | DME Info | Homo sapiens | [4] | |||
| Alanyl aminopeptidase (ANPEP) | DME Info | Homo sapiens | [5] | |||
| Arylsulfatase B (ARSB) | DME Info | Homo sapiens | [4] | |||
| Bile acid-CoA thioesterase (BAAT) | DME Info | Homo sapiens | [4] | |||
| Cytosolic branched aminotransferase (BCAT1) | DME Info | Homo sapiens | [5] | |||
| Catalase (CAT) | DME Info | Homo sapiens | [6] | |||
| Choline dehydrogenase (CHDH) | DME Info | Homo sapiens | [4] | |||
| Cytochrome P450 1A1 (CYP1A1) | DME Info | Homo sapiens | [7], [4] | |||
| Vitamin D(3) 24-hydroxylase (CYP24A1) | DME Info | Homo sapiens | [4] | |||
| Retinoic acid 4-hydroxylase 26B1 (CYP26B1) | DME Info | Homo sapiens | [4] | |||
| Cytochrome P450 3A4 (CYP3A4) | DME Info | Homo sapiens | [8] | |||
| Delta(24)-sterol reductase (DHCR24) | DME Info | Homo sapiens | [5] | |||
| Dihydrothymine dehydrogenase (DPYD) | DME Info | Homo sapiens | [9] | |||
| Eosinophil peroxidase (EPX) | DME Info | Homo sapiens | [5] | |||
| Hexosephosphate aminotransferase 2 (GFPT2) | DME Info | Homo sapiens | [10] | |||
| L-glutamine amidohydrolase (GLS) | DME Info | Homo sapiens | [4] | |||
| Glutathione reductase (GSR) | DME Info | Homo sapiens | [11] | |||
| HMG-CoA reductase (HMGCR) | DME Info | Homo sapiens | [5] | |||
| Iduronate 2-sulfatase (IDS) | DME Info | Homo sapiens | [7] | |||
| Lactoperoxidase (LPO) | DME Info | Homo sapiens | [5] | |||
| Methionine-tRNA ligase mitochondrial (MARS2) | DME Info | Homo sapiens | [5] | |||
| S-adenosylmethionine synthase 2 (MAT2A) | DME Info | Homo sapiens | [5] | |||
| Myeloperoxidase (MPO) | DME Info | Homo sapiens | [5] | |||
| Methylsterol monooxygenase 1 (MSMO1) | DME Info | Homo sapiens | [12], [5] | |||
| Peptide methionine sulfoxide reductase (MSRA) | DME Info | Homo sapiens | [4], [5] | |||
| Mevalonate pyrophosphate decarboxylase (MPD) | DME Info | Homo sapiens | [12], [4] | |||
| Cysteinyl-conjugate N-acetyltransferase (NAT8) | DME Info | Homo sapiens | [4] | |||
| Deoxy-5'-nucleotidase 1 (NT5C) | DME Info | Homo sapiens | [4] | |||
| Phosphodiesterase 5A (PDE5A) | DME Info | Homo sapiens | [4] | |||
| Phosphodiesterase 7B (PDE7B) | DME Info | Homo sapiens | [4] | |||
| Phosphoribosylformylglycinamidine synthase (PFAS) | DME Info | Homo sapiens | [5] | |||
| Cytosolic phospholipase A2 (PLA2G4A) | DME Info | Homo sapiens | [4] | |||
| Prostaglandin G/H synthase 2 (COX-2) | DME Info | Homo sapiens | [13] | |||
| Selenocysteine lyase (SCLY) | DME Info | Homo sapiens | [4] | |||
| Steroid 5-alpha-reductase 3 (SRD5A3) | DME Info | Homo sapiens | [7] | |||
| Sulfotransferase 2B1 (SULT2B1) | DME Info | Homo sapiens | [4] | |||
| Thioredoxin reductase TR1 (TXNRD1) | DME Info | Homo sapiens | [14] | |||
| Thymidine phosphorylase (TYMP) | DME Info | Homo sapiens | [9], [4] | |||
| UDP-glucuronosyltransferase 2A3 (UGT2A3) | DME Info | Homo sapiens | [4] | |||
| Uridine 5'-monophosphate synthase (UMPS) | DME Info | Homo sapiens | [9] | |||
| Uridine phosphorylase 1 (UPP1) | DME Info | Homo sapiens | [4] | |||
| DME(s) Induced by This Xenobiotics | ||||||
| Acetylcholinesterase (ACHE) | DME Info | Homo sapiens | [4] | |||
| Tartrate-resistant acid ATPase (ACP5) | DME Info | Homo sapiens | [5] | |||
| Acetyl-CoA synthetase (ACSS2) | DME Info | Homo sapiens | [5] | |||
| Adenosine aminohydrolase (ADA) | DME Info | Homo sapiens | [4] | |||
| Hydroxyacid-oxoacid transhydrogenase (ADHFE1) | DME Info | Homo sapiens | [4] | |||
| Small intestine reductase (AKR1B10) | DME Info | Homo sapiens | [10], [4] | |||
| Farnesol dehydrogenase (AKR1B15) | DME Info | Homo sapiens | [4] | |||
| Aldo-keto reductase 1C1 (AKR1C1) | DME Info | Homo sapiens | [15], [4] | |||
| Aldo-keto reductase 1C3 (AKR1C3) | DME Info | Homo sapiens | [15] | |||
| Aldo-keto reductase 1D1 (AKR1D1) | DME Info | Homo sapiens | [4] | |||
| Succinic semialdehyde reductase (AKR7A2) | DME Info | Homo sapiens | [5] | |||
| Aldehyde dehydrogenase 5 (ALDHX) | DME Info | Homo sapiens | [4] | |||
| Succinate-semialdehyde dehydrogenase (ALDH5A1) | DME Info | Homo sapiens | [4] | |||
| Arachidonate 5-lipoxygenase (ALOX5) | DME Info | Homo sapiens | [4], [5] | |||
| Arylsulfatase A (ARSA) | DME Info | Homo sapiens | [4] | |||
| Asparagine synthetase (ASNS) | DME Info | Homo sapiens | [5] | |||
| Bleomycin hydrolase (BLMH) | DME Info | Homo sapiens | [4] | |||
| Serine sulfhydrase (CBS) | DME Info | Homo sapiens | [5] | |||
| Carboxylesterase 2 (CES2) | DME Info | Homo sapiens | [4] | |||
| Microsomal cytochrome MCB5 (CYB5A) | DME Info | Homo sapiens | [5] | |||
| Cytochrome P450 1B1 (CYP1B1) | DME Info | Homo sapiens | [7], [5] | |||
| Cytochrome P450 2S1 (CYP2S1) | DME Info | Homo sapiens | [4] | |||
| Lauric acid omega-hydroxylase (CYP4A11) | DME Info | Homo sapiens | [16] | |||
| Leukotriene B4 omega-hydroxylase (CYP4F3) | DME Info | Homo sapiens | [4] | |||
| Dicarbonyl/L-xylulose reductase (DCXR) | DME Info | Homo sapiens | [4] | |||
| Dihydrofolate reductase (DHFR) | DME Info | Homo sapiens | [5] | |||
| Thymidylate kinase (DTYMK) | DME Info | Homo sapiens | [5] | |||
| Bifunctional epoxide hydrolase 2 (EPHX2) | DME Info | Homo sapiens | [4] | |||
| Fatty acid desaturase 1 (FADS1) | DME Info | Homo sapiens | [4], [5] | |||
| Fatty acid desaturase 2 (FADS2) | DME Info | Homo sapiens | [5] | |||
| Friedreich ataxia protein (FXN) | DME Info | Homo sapiens | [17] | |||
| Sodium/potassium-transporting ATPase gamma (FXYD2) | DME Info | Homo sapiens | [4] | |||
| Gamma-Glu-X carboxypeptidase (GGH) | DME Info | Homo sapiens | [5] | |||
| Cellular glutathione peroxidase (GPX1) | DME Info | Homo sapiens | [4] | |||
| Glutathione S-transferase omega-1 (GSTO1) | DME Info | Homo sapiens | [4] | |||
| Glutathione S-transferase pi (GSTP1) | DME Info | Homo sapiens | [4] | |||
| N-acetyl-beta-glucosaminidase beta (HEXB) | DME Info | Homo sapiens | [5] | |||
| Heparanase (HPSE) | DME Info | Homo sapiens | [4] | |||
| Keto-steroid reductase (HSD17B7) | DME Info | Homo sapiens | [4], [5] | |||
| Indoleamine 2,3-dioxygenase 1 (IDO1) | DME Info | Homo sapiens | [4] | |||
| Alpha-L-iduronidase (IDUA) | DME Info | Homo sapiens | [4] | |||
| Methionine adenosyltransferase II beta (MAT2B) | DME Info | Homo sapiens | [4] | |||
| Microsomal glutathione S-transferase 2 (MGST2) | DME Info | Homo sapiens | [4] | |||
| Metallothionein-1A (MT1A) | DME Info | Homo sapiens | [18] | |||
| Metallothionein-2A (MT2A) | DME Info | Homo sapiens | [7], [18] | |||
| Glutamine-dependent NAD(+) synthetase (NADSYN1) | DME Info | Homo sapiens | [4] | |||
| NADH-ubiquinone oxidoreductase 30 kDa (NDUFS3) | DME Info | Homo sapiens | [4] | |||
| Nitric oxide synthase inducible (NOS2) | DME Info | Homo sapiens | [19] | |||
| Quinone reductase 1 (NQO1) | DME Info | Homo sapiens | [20] | |||
| Purine nucleoside phosphorylase (PNP) | DME Info | Homo sapiens | [4] | |||
| Pyridoxamine-phosphate oxidase (PNPO) | DME Info | Homo sapiens | [4] | |||
| Proline dehydrogenase 1 (PRODH) | DME Info | Homo sapiens | [4] | |||
| Prostaglandin G/H synthase 1 (COX-1) | DME Info | Homo sapiens | [5] | |||
| Putrescine acetyltransferase (SSAT1) | DME Info | Homo sapiens | [10], [5] | |||
| Superoxide dismutase 1 (SOD1) | DME Info | Homo sapiens | [21] | |||
| Transglutaminase K (TGM1) | DME Info | Homo sapiens | [4] | |||
| Transglutaminase X (TGM5) | DME Info | Homo sapiens | [5] | |||
| Thiopurine methyltransferase (TPMT) | DME Info | Homo sapiens | [5] | |||
| Thymidylate synthase (TYMS) | DME Info | Homo sapiens | [9] | |||
| Xanthine dehydrogenase/oxidase (XDH) | DME Info | Homo sapiens | [4] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

