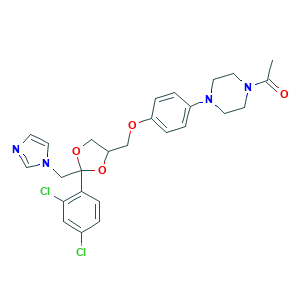
| Synonyms |
Ketoconazol; Ketoconazolum; Ketoderm; Nizoral; Ketoconazole; R 41400; Fungarest; Fungoral; KW-1414; 1-[4-[4-[[2-(2,4-dichlorophenyl)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]piperazin-1-yl]ethanone; 65277-42-1; 79156-75-5; CHEBI:48339; NSC317629; cis-1-Acetyl-4-(4-((2-(2,4-dichlorophenyl)-2-(1H-1M-idazol-1-ylmethyl)-1,3-dioxolan-4-yl) methoxy) phenyl)-piperazine; piperazine, 1-acetyl-4-[4-[[2-(2,4-dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-
|
| Cross-matching ID |
- PubChem CID
- 3823
- PubChem SID
-
456469
; 5316931
; 8152421
; 11407121
; 29222942
; 46506746
; 46530753
; 47261387
; 51021382
; 57322009
; 85087144
; 85174349
; 85787593
; 93620728
; 103845251
; 104014719
; 104304628
; 117361105
; 117571495
; 118836543
; 125355519
; 125357821
; 125433866
; 125544749
; 126685446
; 126728346
; 127316656
; 127316657
; 127316658
; 127316659
; 127316660
; 129708236
; 135650454
; 137100792
; 142426911
; 143837446
; 152057681
; 162022954
; 162092001
; 162914824
; 163315720
; 164811453
; 164830408
; 172824381
; 179116748
; 223677862
; 223726587
; 226399613
; 252825836
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0B4IF
- Formula
- C26H28Cl2N4O4
- Canonical SMILES
- CC(=O)N1CCN(CC1)C2=CC=C(C=C2)OCC3COC(O3)(CN4C=CN=C4)C5=C(C=C(C=C5)Cl)Cl
- InChI
- 1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3
- InChIKey
- XMAYWYJOQHXEEK-UHFFFAOYSA-N
|