| Cross-matching ID |
- PubChem CID
- 65981
- PubChem SID
-
9872
; 7847660
; 7980507
; 8190080
; 11112894
; 11467074
; 11468194
; 11486642
; 12014667
; 14808699
; 14833410
; 24724596
; 26719812
; 43122707
; 46386564
; 46508150
; 47425572
; 48244693
; 48319772
; 48395177
; 48416511
; 49648522
; 49699266
; 49888451
; 50100536
; 52108264
; 53789672
; 57315871
; 81092844
; 85788088
; 92125938
; 92308165
; 92308498
; 92719265
; 93166496
; 99218180
; 99431925
; 99437146
; 103394824
; 103914669
; 104041348
; 104179029
; 104335174
; 117545849
; 117873275
; 121363609
; 124659000
; 124757239
; 124799919
; 125164043
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0N5YA
- Formula
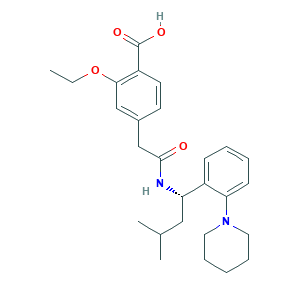
- C27H36N2O4
- Canonical SMILES
- CCOC1=C(C=CC(=C1)CC(=O)NC(CC(C)C)C2=CC=CC=C2N3CCCCC3)C(=O)O
- InChI
- 1S/C27H36N2O4/c1-4-33-25-17-20(12-13-22(25)27(31)32)18-26(30)28-23(16-19(2)3)21-10-6-7-11-24(21)29-14-8-5-9-15-29/h6-7,10-13,17,19,23H,4-5,8-9,14-16,18H2,1-3H3,(H,28,30)(H,31,32)/t23-/m0/s1
- InChIKey
- FAEKWTJYAYMJKF-QHCPKHFHSA-N
|