| General Information of Drug (ID:
DR1500) |
| Drug Name |
Sorafenib
|
| Synonyms |
Sorafenib; Nexavar; 284461-73-0; 4-(4-(3-(4-CHLORO-3-(TRIFLUOROMETHYL)PHENYL)UREIDO)PHENOXY)-N-METHYLPICOLINAMIDE; 4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)phenoxy]-N-methylpyridine-2-carboxamide; 4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]phenoxy]-N-methylpyridine-2-carboxamide; 9ZOQ3TZI87; BAY 43-9006; CHEMBL1336; N-(4-Chloro-3-(trifluoromethyl)phenyl)-N'-(4-(2-(N-methylcarbamoyl)-4-pyridyloxy)phenyl)urea; UNII-9ZOQ3TZI87
|
| Indication |
Renal cell carcinoma
[ICD11: 2C90]
|
Approved
|
[1]
|
| Structure |
|
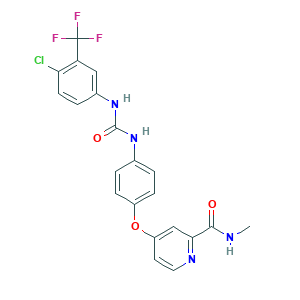 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
464.8 |
Topological Polar Surface Area |
92.4 |
| Heavy Atom Count |
32 |
Rotatable Bond Count |
5 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
7 |
| Cross-matching ID |
- PubChem CID
- 216239
- PubChem SID
-
833589
; 833590
; 7886069
; 9372814
; 12015507
; 14720365
; 14834034
; 30419984
; 46505329
; 46516901
; 50069824
; 50070560
; 50071324
; 50100118
; 50112741
; 53787819
; 53799234
; 56312334
; 56312336
; 56312338
; 56312517
; 56312519
; 56312521
; 56394953
; 57399755
; 68529952
; 74382940
; 75518900
; 81092852
; 85174603
; 85285882
; 85845166
; 91148447
; 92718861
; 93581025
; 96025209
; 99443909
; 103420820
; 103904444
; 104178872
; 113461200
; 117695450
; 117871124
; 124767621
; 124893320
; 124893321
; 124893322
; 124893323
; 124893324
; 125346960
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0W5HK
- Formula
- C21H16ClF3N4O3
- Canonical SMILES
- CNC(=O)C1=NC=CC(=C1)OC2=CC=C(C=C2)NC(=O)NC3=CC(=C(C=C3)Cl)C(F)(F)F
- InChI
- 1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31)
- InChIKey
- MLDQJTXFUGDVEO-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


