| General Information of Drug (ID:
DR1509) |
| Drug Name |
Sulfadiazine
|
| Synonyms |
Sanodiazine; Spofadrizine; Sterazine; Sulfadiazene; Sulfadiazin; Sulfanilamidopyrimidine; Sulfapyrimidin; Sulfapyrimidine; Sulfatryl; Sulfazine; Sulfolex; Sulfonsol; Sulfose; Sulphadiazine; Terfonyl; Theradiazine; Tri-Sulfameth; Trifonamide; Adiazin; Adiazine; Cremodiazine; Cremotres; Debenal; Deltazina; Diazin; Diazolone; Diazyl; Eskadiazine; Honey diazine; Lipo-Levazine; Liquadiazine; Microsulfon; Neazine; Neotrizine; Palatrize; Piridisir; Pirimal; Pyrimal; Quadetts; Quadramoid; Trisem; Truozine; sulfadiazine; 2-Sulfanilamidopyrimidine; 68-35-9
|
| Indication |
Toxoplasmosis
[ICD11: 1F57]
|
Approved
|
[1]
|
| Structure |
|
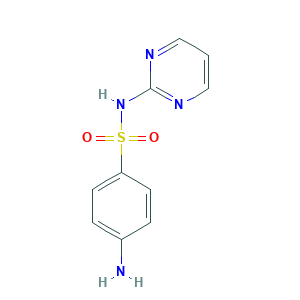 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
250.28 |
Topological Polar Surface Area |
106 |
| Heavy Atom Count |
17 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
6 |
| Cross-matching ID |
- PubChem CID
- 5215
- PubChem SID
-
9860
; 92611
; 602777
; 642441
; 642967
; 855872
; 933844
; 3182910
; 4484320
; 7847653
; 7980699
; 8149533
; 8153205
; 10321441
; 10532663
; 11112193
; 11335718
; 11360957
; 11363880
; 11366442
; 11369004
; 11372203
; 11373882
; 11377166
; 11461929
; 11466051
; 11467171
; 11485153
; 11485742
; 11489135
; 11491177
; 11492157
; 11494800
; 11533903
; 14749834
; 24861734
; 24899802
; 25623038
; 26611925
; 26679790
; 26747022
; 26747023
; 29224272
; 46506164
; 47216749
; 47440219
; 47588965
; 47662250
; 47736446
; 47885378
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D05LKP
- Formula
- C10H10N4O2S
- Canonical SMILES
- C1=CN=C(N=C1)NS(=O)(=O)C2=CC=C(C=C2)N
- InChI
- 1S/C10H10N4O2S/c11-8-2-4-9(5-3-8)17(15,16)14-10-12-6-1-7-13-10/h1-7H,11H2,(H,12,13,14)
- InChIKey
- SEEPANYCNGTZFQ-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


