| Synonyms |
Cabotegravir; Cabotegravir (GSK744, GSK1265744); Cabotegravir [USAN:INN]; S/GSK1265744; 1051375-10-0; CAB; GSK-1265744; GSK1265744; GSK1265744A; GSK744; GSK744 (S/GSK1265744); GSK744 LA; GSK744 LAP; HMH0132Z1Q; Oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide, N-[(2,4-difluorophenyl)methyl]-2,3,5,7,11,11a-hexahydro-6-hydroxy-3-methyl-5,7-dioxo-, (3S,11aR)-;Oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide, N-[(2,4-difluorophenyl)methyl]-2,3,5,7,11,11a-hexahydro-6-hydroxy-3-methyl-5,7-dioxo-, (3S,11aR)-; UNII-HMH0132Z1Q
|
| Cross-matching ID |
- PubChem CID
- 54713659
- PubChem SID
-
17424254
; 36462227
; 76221742
; 138088923
; 162108745
; 174502414
; 198940204
; 198993890
; 226461534
; 244829189
; 249732325
- CAS Number
-
- TTD Drug ID
- D08LDB
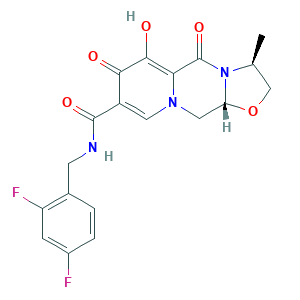
- Formula
- C19H17F2N3O5
- Canonical SMILES
- CC1COC2N1C(=O)C3=C(C(=O)C(=CN3C2)C(=O)NCC4=C(C=C(C=C4)F)F)O
- InChI
- 1S/C19H17F2N3O5/c1-9-8-29-14-7-23-6-12(16(25)17(26)15(23)19(28)24(9)14)18(27)22-5-10-2-3-11(20)4-13(10)21/h2-4,6,9,14,26H,5,7-8H2,1H3,(H,22,27)/t9-,14+/m0/s1
- InChIKey
- WCWSTNLSLKSJPK-LKFCYVNXSA-N
|