| Synonyms |
Beta-(p-Hydroxyphenyl)alanine; Free-Form L-Tyrosine; L-(-)-Tyrosine; L-Tyrosin; L-Tyrosine (9CI); L-Tyrosine (JAN); L-Tyrosine hydrochloride; L-Tyrosine, homopolymer; L-Tyrosine, monomer; L-p-Tyrosine; P-Tyrosine; Rxosine; TYR NH3+ COOH; Tirosina; Tirosina [Spanish]; Tyrosine; Tyrosine (USP/INN); Tyrosine (VAN); Tyrosine Power; Tyrosine [USAN:INN]; Tyrosine, L-(8CI); Tyrosinum; Tyrosinum [Latin]; (-)-alpha-Amino-p-hydroxyhydrocinnamic acid; (S)-2-Amino-3-(4-hydroxyphenyl)propionic acid; (S)-2-Amino-3-(p-hydroxyphenyl)propionic acid; (S)-3-(p-Hydroxyphenyl)alanine; (S)-Tyrosine; (S)-alpha-Amino-4-hydroxybenzenepropanoic acid; 2-Amino-3-(p-hydroxyphenyl)propionic acid; 3-(4-Hydroxyphenyl)-L-alanine; 3-(p-Hydroxyphenyl)alanine; 4-Hydroxy-L-phenylalanine; 4ts1; Alpha-Amino-beta-(4-hydroxyphenyl)propionic acid; DD69927C-C6A8-4BC6-8E9A-0AB423B176E7; DTY; H-Tyr-OH; L-2-Amino-3-p-hydroxyphenylpropanoic acid; Melanin synthesized from Tyr substrate catalyzed by tyrosinase for 6 hrs; Melanin synthesized from Tyr substrate catalyzed by tyrosinase for 6 hrs, oxidized with hydrogen peroxide; Melanin synthesized from Tyr substrate catalyzed by tyrosinase for 6 hrs, oxidized with hydrogen peroxide, <3 kd fraction; Melanin synthesized from Tyr substrate catalyzed by tyrosinase, brominated with N-bromosuccinimide; Melanin synthesized from Tyr substrate catalyzed by tyrosinase, sulfonated using sulfur trioxide/DMF complex for 1.5-7 hours; Tyr
|
| Cross-matching ID |
- PubChem CID
- 6057
- PubChem SID
-
3382
; 585619
; 585656
; 608132
; 817482
; 817486
; 817487
; 817488
; 817489
; 823160
; 828554
; 832231
; 841729
; 854649
; 3134840
; 7847090
; 7887220
; 7890924
; 8026808
; 8028352
; 8028353
; 8144790
; 8153780
; 10529154
; 11528398
; 14710671
; 15147336
; 15195185
; 24697644
; 24697646
; 24889931
; 24900164
; 24900206
; 24900543
; 24901863
; 25622129
; 26702499
; 26711663
; 26711664
; 26711665
; 26718889
; 26719042
; 29215265
; 29215266
; 29225067
; 46394031
; 46394400
; 46504669
; 46507885
; 48416689
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D01CRB
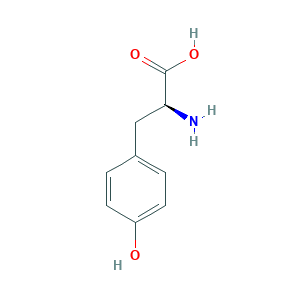
- Formula
- C9H11NO3
- Canonical SMILES
- C1=CC(=CC=C1CC(C(=O)O)N)O
- InChI
- 1S/C9H11NO3/c10-8(9(12)13)5-6-1-3-7(11)4-2-6/h1-4,8,11H,5,10H2,(H,12,13)/t8-/m0/s1
- InChIKey
- OUYCCCASQSFEME-QMMMGPOBSA-N
|