| Cross-matching ID |
- PubChem CID
- 222528
- PubChem SID
-
7102
; 74413
; 585592
; 585594
; 618558
; 819839
; 828927
; 837869
; 841387
; 855438
; 3135058
; 7850928
; 7887027
; 7887240
; 8138505
; 9377073
; 11446298
; 14717701
; 15953970
; 24277530
; 24858743
; 24893692
; 24893857
; 25622261
; 29204024
; 30425733
; 46391767
; 47193711
; 47959957
; 50086825
; 53777021
; 53786947
; 56311772
; 57399984
; 80526818
; 85279390
; 90451786
; 93167216
; 99444161
; 99455580
; 103578732
; 113476161
; 124807327
; 124812927
; 125011664
; 125308743
; 126523430
; 126596873
; 126606721
; 127310875
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D02PQB
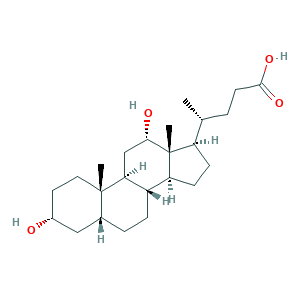
- Formula
- C24H40O4
- Canonical SMILES
- CC(CCC(=O)O)C1CCC2C1(C(CC3C2CCC4C3(CCC(C4)O)C)O)C
- InChI
- 1S/C24H40O4/c1-14(4-9-22(27)28)18-7-8-19-17-6-5-15-12-16(25)10-11-23(15,2)20(17)13-21(26)24(18,19)3/h14-21,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15-,16-,17+,18-,19+,20+,21+,23+,24-/m1/s1
- InChIKey
- KXGVEGMKQFWNSR-LLQZFEROSA-N
|